Translate this page into:
Subungual hyperkeratosis nail biopsy: A better diagnostic tool for onychomycosis
2 Department of Microbiology, T.N.M.C. Medical College and B.Y.L. Nair Hospital, Mumbai, India
Correspondence Address:
Chitra S Nayak
Department of Skin and V.D, T.N.M.C. Medical College and, B. Y. L. Nair Charitable Hospital, Dr. A. L. Nair road, Mumbai - 400 008
India
| How to cite this article: Nagar R, Nayak CS, Deshpande S, Gadkari RP, Shastri J. Subungual hyperkeratosis nail biopsy: A better diagnostic tool for onychomycosis. Indian J Dermatol Venereol Leprol 2012;78:620-624 |
Abstract
Background: Onychomycosis is one of the most common nail disorders. Mycological examination by potassium hydroxide (KOH) mount and fungal culture is the most commonly used diagnostic method. However, it is associated with a low sensitivity. Aims: To evaluate the technique of subungual hyperkeratosis nail biopsy in diagnosing onychomycosis in HIV-infected and immunocompetent adults and compare it with mycological examination. Methods: 34 HIV-positive patients who presented clinically with onychomycosis were recruited in the study from the beginning. There was no screening done for patients with onychomycosis. This has been clarified in manuscript under the heading of methods. Results: All the fungal cultures yielded dermatophytes correlating with the biopsy findings. Only hyphal form of fungus was detected in KOH examination, indicating it was not a contaminant. Clinical types of onychomycosis are stated in discussion. Conclusions: PAS stain of subungual hyperkeratosis nail biopsy was the most sensitive in the diagnosis of onychomycosis in both HIV-infected and non-infected groups.Introduction
About 50% of dystrophic nails have a fungal etiology, [1] making it imperative to establish a correct and prompt laboratory diagnosis before instituting an anti-fungal therapy. A false negative diagnosis may lead to continued disfigurement and discomfort as well as repeated attacks of fungal infections elsewhere on the body, whereas a false positive diagnosis may lead to an unnecessary, long, and expensive treatment. [2] The incidence of onychomycosis has increased progressively during the last few years. HIV infection is a risk factor for the development of onychomycosis. Up to 30% to 78% of HIV-infected patients are said to have onychomycosis. [3] These patients are usually on multiple medications including anti-retroviral drugs; therefore, a correct diagnosis is needed in them before starting systemic anti-fungal drugs, which are known to cause multiple drug interactions and adverse effects. A KOH mount is a useful screening test, but culture is considered the mainstay of diagnosis because it not only has high sensitivity, but also allows identification of the etiologic agent, so that treatment may be appropriately tailored. However, negative results are frequent, and the diagnosis may be delayed up to 6 weeks. Histological examination of nail clippings with PAS staining is the most sensitive diagnostic test for onychomycosis. [4],[5] But, processing of nail clippings is technically complex and expensive. Chang described a modified technique of diagnosing onychomycosis using subungual hyperkeratosis in 2007. [5] It was found to be more sensitive, less costly, and technically easier. The current study was undertaken to evaluate this modified technique in detecting onychomycosis in HIV-infected and non-infected patients with nail changes suggestive of onychomycosis and to compare its sensitivity with the standard diagnostic tests viz. mycological examination.
Methods
The study was conducted for a period of 1 year between September 2008 and August 2009 in the dermatology department of a tertiary care institute. 34 (50%) HIV-positive adult patients (irrespective of their CD4 count and anti-retroviral treatment) and 34 (50%) HIV-negative adult patients who presented clinically with distal and lateral subungual onychomycosis and adequate amount of subungual hyperkeratotic material [Figure - 1] and who had not taken anti-fungal treatment for the present condition were included in the study. Patients suffering from onychodystrophy due to other dermatological conditions such as psoriasis, lichen planus, eczema, candidial paronychia, and onychomycosis due to non-dermatophyte moulds were excluded from the study. A written consent was obtained from each patient prior to enrolment, and the study was carried out after obtaining the institutional ethics committee approval.
The affected nails were cleaned with 70% alcohol to remove any potential contaminants. Depending on the thickness of the nail plate and the amount of subungual hyperkeratotic material, either a nail clipper with a long handle and short cutting edges, or a bone nibbler was used. The nails were clipped as close to the proximal nail fold without causing any damage to the underlying nail bed so as to obtain maximum possible subungual hyperkeratotic material. The subungual hyperkeratotic material attached to the nail plate was removed using a no. 11 surgical blade and was divided into 2 equal parts. One part was fixed in 10% formalin and sent for histological examination. It was processed in a similar manner as normal skin biopsies and embedded in paraffin. hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) stains were performed on the sections. Presence of intensely eosinophilic fungal elements on PAS stain [Figure - 2] and hyaline-like fungal elements on H&E stain [Figure - 3] on high power (40×) was considered as a positive finding.
The other part of the subungual hyperkeratotic material was subjected to mycological examination. Microscopic examination was carried out by using 20% KOH mount. A few drops of 20% KOH were put on a clean grease-free slide, and a small portion of the nail specimen was added to it and covered with a cover slip. After overnight incubation, the slides were examined for the presence of fungal elements under the high power of microscope [Figure - 4]. For culture, the remaining specimen was inoculated on two Sabouraud′s agar slants containing cycloheximide and chloramphenicol. The Sabouraud′s agar slants were incubated at 37°C and room temperature. The slants were examined at regular intervals for growth [Figure - 5]. No growth was reported if colonies did not appear in 4 weeks. The results were tabulated, and statistical evaluation was done using Chi-square test.
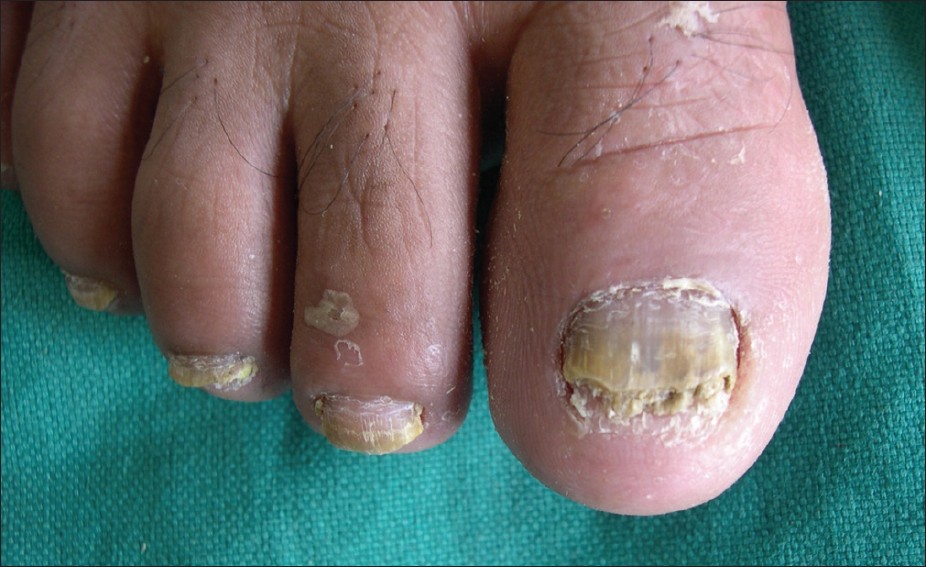 |
| Figure 1: Onychomycosis with abundant subungual hyperkeratosis |
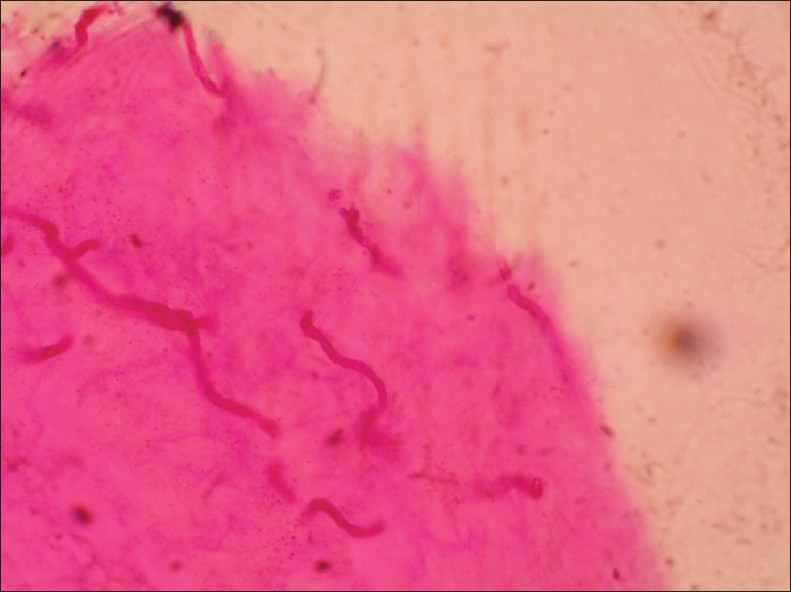 |
| Figure 2: Eosinophilic fungal hyphae (PAS, ×40) |
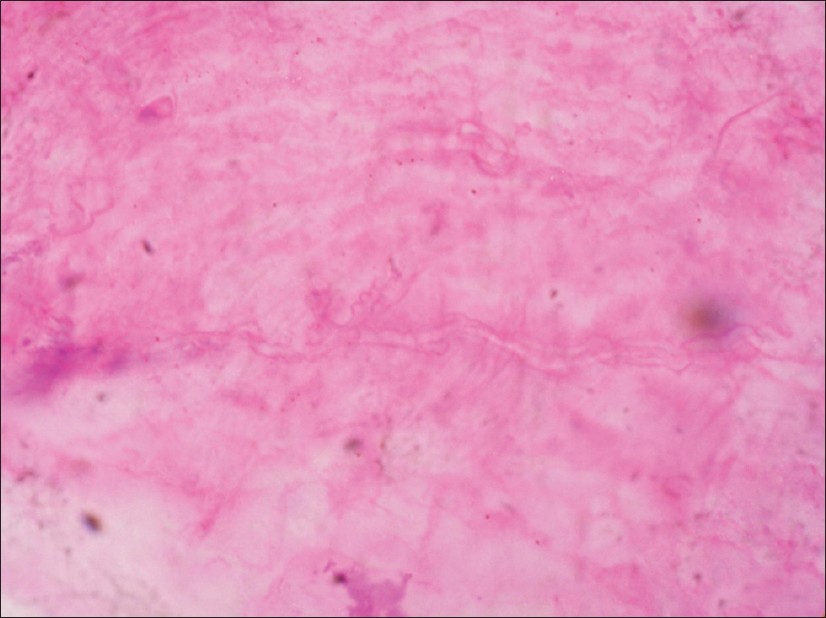 |
| Figure 3: Hyaline fungal hyphae (H&E, ×40) |
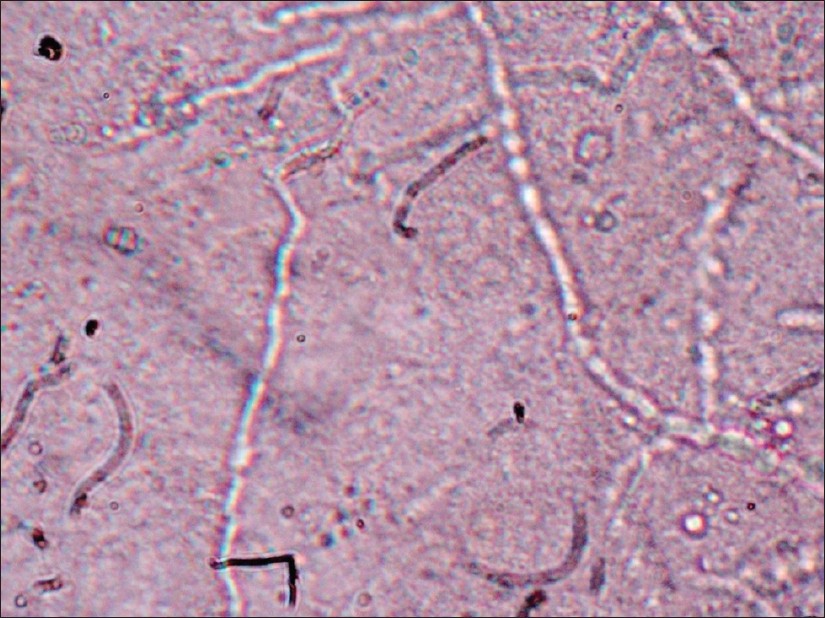 |
| Figure 4: Hyaline, septate hyphae diagnostic for a dermatophyte (KOH mount, ×40) |
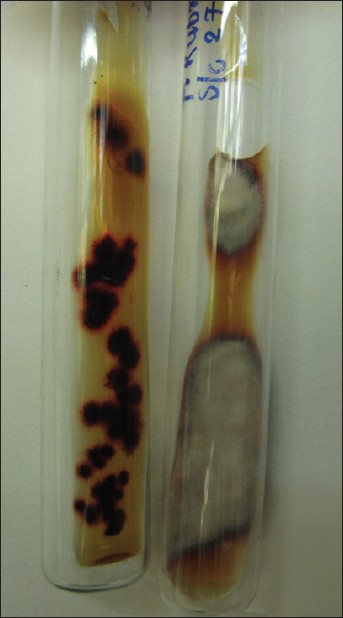 |
| Figure 5: Sabouraud's dextrose agar shows growth of T. rubrum |
Results
Amongst the 34 HIV-infected patients, PAS stained subungual hyperkeratosis of nail biopsy was positive in 31 (91%) and hematoxylin and eosin (H&E) stained in 18 (53%) patients. Mycological examination was positive in 26 (76%) patients and culture in 12 (35%) patients. In the 34 patients from control group (non-HIV positive), the corresponding figures were 30 (88%), 14 (41%), 17 (50%), and 10 (29%) respectively, [Table - 1].

A statistical analysis of HIV infection status as a variable with respect to results on periodic acid-Schiff (PAS) stain, H&E stain, and mycological examination results was performed. Our study did not find any significant association between HIV infection status and PAS results (corrected Chi square = 1, P < 0.05). Similarly, no significant association was found between HIV infection status and H&E results (corrected Chi-square = 0.466, P < 0.05). However, the difference in the positive results of mycological examination between the HIV-infected and non-HIV positive groups (corrected Chi- square = 0.044) was statistically significant (P < 0.05).
A comparative analysis of PAS stained subungual hyperkeratosis nail biopsy with mycological examination in the HIV-infected group revealed that both the techniques were positive in 24 cases. PAS was positive in additional 7 cases, and mycological examination in 2 cases. Only 1 case was missed by both the techniques. On comparing H&E results with mycological examination, it was seen that both the techniques were able to diagnose 14 cases. H&E could diagnose only 4 additional cases, but failed to detect 12 cases, which were otherwise diagnosed by mycological examination. Both the techniques failed to diagnose 4 cases [Table - 2]. Thus, histological examination of subungual hyperkeratosis with PAS stain was most sensitive while H&E staining was least sensitive.

A similar analysis of PAS stained biopsy with mycological examination, in the non-HIV positive group, showed that the 2 techniques could together diagnose 16 cases. PAS diagnosed a substantial number of 14 additional cases, which otherwise would have been falsely reported as negative. Mycological examination was successful in diagnosing only 1 extra case. 3 cases could not be diagnosed by either of the 2 techniques. On comparing H&E stained biopsy results with mycological examination, it was found that both the techniques were positive in 6 cases and negative in 9 cases. H&E could diagnose 8 additional cases while mycological examination diagnosed 11 extra cases [Table - 3]. Histological examination of subungual hyperkeratosis with PAS stain was the most sensitive diagnostic technique for the HIV non-infected group as well, just like the HIV-infected group.

Discussion
Onychomycosis is not life-threatening, but it can cause pain, discomfort and disfigurement. Psychosocial and emotional effects resulting from onychomycosis are widespread and may have a significant impact on quality of life. [6] Nail symptoms are much more frequent in HIV-infected patients than in healthy controls. [3] A correct diagnosis is of utmost importance before initiating therapy, which is not only of long duration and costly but also potentially toxic. The different clinical types of onychomycosis are distal and lateral subungual onychomycosis (most common type), white superficial onychomycosis, and proximal subungual onychomycosis.
Mycological diagnosis of onychomycosis, which is the most common in vitro method of diagnosis, consists of detection of fungal elements by KOH mount and identification of the responsible fungus by culture. KOH mount is a simple, rapid, inexpensive test to perform, which requires minimum infrastructure but requires considerable experience and expertise as it may result in a high number of false negatives. Culture is the only method that can identify the genus and species of the causative pathogen. It also can differentiate between viable and non-viable fungi. However, the major drawbacks are inoculating in a suitable media and incubating at correct temperature, frequent contamination prompting repeated sampling, delayed results, variable sensitivity and greater cost as compared to KOH mount. The sensitivity ranges from 25% to 80% with an approximately 30% false negative results with KOH and culture. [7] KOH preparations and fungal cultures have limited sensitivities for the diagnosis. From patients with clinically-suspected fungal nail infections, 54.5% had onychomycosis by culture and/or direct microscopic examination in a study conducted at Delhi. [8] The result in our study was comparable as 50% in the non-HIV infected group but was higher (76%) in the HIV-infected group, and this difference was statistically significant.
Histological examination of nail clippings with PAS staining is the most sensitive diagnostic test for onychomycosis. [4] PAS stain demonstrates the presence of certain polysaccharides present in the walls of fungal elements. However, difficulties in processing nail plates limit its use. Processing nail clippings using the same method as skin biopsies makes it difficult to cut the hard keratin of the thickened nail plate in paraffin by a microtome. [9],[10] Nail plate may get dislodged and lost from the paraffin block during processing owing to this hardness. [11] Various types of techniques for nail processing and softening agents such as 10% urea, 3% phenol, 5 and 10% Tween 40, [12] 5% trichloroacetic acid in 10% formalin, KOH followed by a solution of detergent and ammonia and cedar oil [9] have been described in literature. This suggests that in most instances, special techniques are needed to produce adequate sections for histological analysis.
In onychomycosis, subungual hyperkeratosis contains more fungal elements than the nail plate itself. Nail clippings target the nail plate instead of subungual hyperkeratosis. Chang et al, in 2007, described a modified approach to the histological examination of onychomycosis based on examination of subungual hyperkeratosis. [5] By bypassing the difficulties associated with processing the hard keratin of the nail plate, the diagnosis of onychomycosis could be made more efficiently and at lower cost.
Our study was conducted to evaluate this relatively new technique for diagnosing onychomycosis and comparing it with mycological examination in 2 groups of patients viz. the HIV-infected and HIV non-infected. The most sensitive technique was PAS stained subungual hyperkeratosis nail biopsy in both the groups with detection rates averaging around 90%. This was in comparison to 97% detection rate in the study conducted by Chang et al, [5] Histological examination with H&E stain was the least sensitive technique in both the groups. The low sensitivity of H&E stain could be due to lack of experience in distinguishing hyaline appearing fungal elements.
This technique cannot be used in cases of white superficial onychomycosis where the nail plate is mainly involved.
A correct diagnosis of onychomycosis depends on various factors such as adequacy of specimen collection, expertise of the laboratory staff, and skill in the interpretation of results.
Conclusion
Since negative mycology does not completely rule out onychomycosis, the technique of subungual hyperkeratosis nail biopsy with PAS stain is very useful in detecting onychomycosis in both HIV-infected and non-infected patients; therefore, it should be routinely employed.
Acknowledgement
Reseach Society, B.Y.L. Nair Hospital, Mumbai
| 1. |
Scher RK. Current therapy. In: Conn H, editor. Diseases of the nails. Philadelphia: WB Saunders; 1990. p. 736.
[Google Scholar]
|
| 2. |
Ellis DH, Watson A. Management of cutaneous fungal infections. Australian Prescriber 1996;19:72-5.
[Google Scholar]
|
| 3. |
Cribier B, Mena ML, Rey D. Partisani M, Fabien V, Lang JM, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol 1998;134:1216-20.
[Google Scholar]
|
| 4. |
Mahoney JM, Bennet J, Olsen B. The diagnosis of onychomycosis. Dermatol Clin 2003;21:463-7.
[Google Scholar]
|
| 5. |
Chang A, Wharton J, Tam S, Kovich OI, Kamino H. A modified approach to the histologic diagnosis of onychomycosis. J Am Acad Dermatol 2007;57:849-55.
[Google Scholar]
|
| 6. |
Lubeck DP. Measuring health-related quality of life in onychomycosis. J Am Acad Dermatol 1998;38:64-83.
[Google Scholar]
|
| 7. |
Lawry MA, Haneke E, Strobeck K, Martin S, Zimmer B, Romano PS. Methods for diagnosing onychomycosis. Arch Dermatol 2000;136:1112-6.
[Google Scholar]
|
| 8. |
Kaur R, Kashyap B, Bhalla P. A five-year survey of onychomycosis in New Delhi, India: Epidemiological and laboratory aspects. Indian J Dermatol 2007;52:39-42.
[Google Scholar]
|
| 9. |
Fleckman P, Omura EF. Histopathology of the nail. Adv Dermatol 2001;17:385-406.
[Google Scholar]
|
| 10. |
Omura EF. Histopathology of the nail. Dermatol Clin 1985;3:531- 41.
[Google Scholar]
|
| 11. |
Mondragon G. Histotechnologist to histopathologist: A method for processing specimens of nails. Dermatopathol Pract Conceptual 1996;2:41-2.
[Google Scholar]
|
| 12. |
Pierard GE, Arrese JE, De Doncker P, Pierard-Franchimont C. Present and potential diagnostic techniques in onychomycosis. J Am Acad Dermatol 1996;34:273-7.
[Google Scholar]
|
Fulltext Views
10,453
PDF downloads
2,186





