Successful treatment of two cases of refractory cutaneous lupus erythematosus with belimumab
Corresponding author: Dr. José M. Mascaró Jr, Department of Dermatology, Hospital Clínic – IDIBAPS, University of Barcelona, Villarroel Street 170, 08036, Barcelona, Spain. jmmascaro_galy@ub.edu
-
Received: ,
Accepted: ,
How to cite this article: Bosch-Amate X, Morgado-Carrasco D, Combalia A, Giavedoni P, Espinosa G, Mascaró Jr JM. Successful treatment of two cases of refractory cutaneous lupus erythematosus with belimumab. Indian J Dermatol Venereol Leprol 2021;87:421-4.
Sir,
Cutaneous lupus erythematosus is an autoimmune skin disorder that may be associated with systemic lupus erythematosus. Treatment of cutaneous lupus erythematosus includes photoprotection, topical corticosteroids and calcineurin inhibitors, systemic agents such as antimalarials, retinoids, corticosteroids and other immunosuppressants. Refractory cases and intolerance to first-line systemic therapies present challenging situations.1
Belimumab is the first drug specifically approved for systemic lupus erythematosus in more than 50 years,2 with good clinical response. However, the evidence about its effectiveness in cutaneous lupus erythematosus not associated with systemic lupus erythematosus is scarce. Herein, we describe two patients with refractory cutaneous lupus erythematosus that did not fulfil systemic lupus erythematosus criteria, and at the same time, showed an excellent response to belimumab.
The first case was a 47-year old man with an 8-year history of extensive and severe biopsy-proven chronic discoid lupus erythematosus lesions on the scalp. Blood tests revealed positive antinuclear antibodies (1:640, homogeneous-speckled pattern) and low complement levels (C3 0.45 g/L, normal value >0.82 g/L; C4 0.07 g/L, normal value >0.11 g/L; CH50 24 U/mL, normal range 34-71 U/mL). The remaining antibodies (anti-double stranded DNA, anti-Sm, anti-U1-RNP, anti-Ro/SSA and anti-La/SSB) were repeatedly negative. Renal function parameters and blood counts were normal. Hepatic enzymes were elevated due to a concomitant autoimmune hepatitis. The patient did not fulfill systemic lupus erythematosus criteria (only two out of four of the American College of Rheumatology (ACR) criteria were positive- presence of antinuclear antibodies and discoid rash). He had received multiple systemic treatment (including prednisone up to 90 mg/day, hydroxychloroquine 400 mg/day, azathioprine 150 mg/day, mycophenolic acid 720 mg twice a day and isotretinoin 20 mg/day) without clinical improvement [Table 1]. Before starting belimumab, he was on prednisone 15 mg/ day, hydroxychloroquine 400 mg/day and mycophenolic acid 720 mg twice a day but had still cutaneous flares and skin infections. His cutaneous lupus erythematosus disease area and severity index (CLASI) activity score was 16 [Figure 1a].
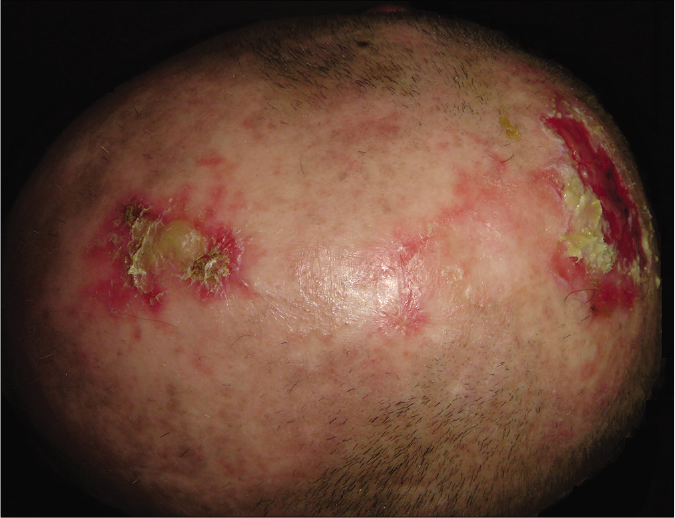
- Patient 1: Crusted plaques and erosions of chronic discoid lupus erythematosus on scalp
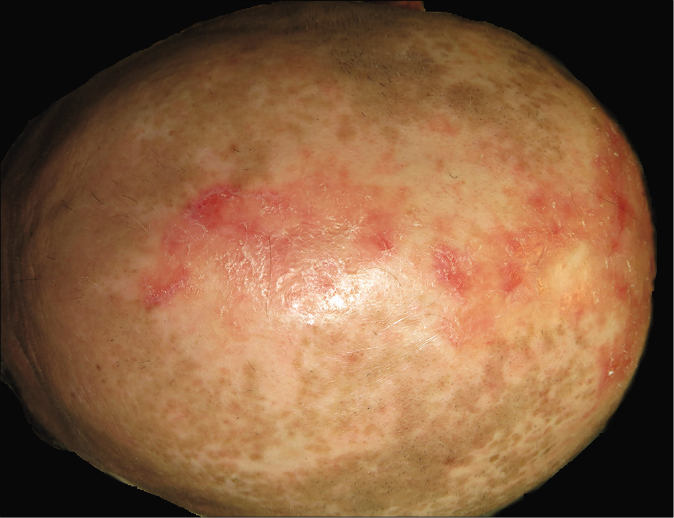
- Patient 1: Resolution of cutaneous lesions with belimumab (4 months after initiation)
| Case/sex/age | Clinical features | American College of Rheumatology criteria | Previous treatments | CLASI score activity before belimumab treatment | CLASI score activity after belimumab treatment | Follow-up |
|---|---|---|---|---|---|---|
| 1/male/47 | Plaques and erosions on face and scalp Multiple skin infections of the lesions |
Discoid rash Positive antinuclear antibodies |
Prednisone up to 90 mg/day for 8 years concomitantly with all the others treatments. Hydroxychloroquine 400 mg/day for 8 years concomitantly with all the others treatments. Azathioprine 150 mg/day for 4 years. Mycophenolic acid up to 720 mg twice a day for 4 years sequentially to azathioprine. Isotretinoin 20 mg/day for 6 month concomitantly with prednisone, hydroxychloroquine and mycophenolic acid. |
16: Pink erythema on ears (1), posterior neck and shoulders (1); red erythema on rest of the face (2); dark red erythema on scalp (3), hands (3); hypertrophic lesions on hands (2); recent hair loss (1); and focal alopecia in more than one quadrant (3) | 0 | Asymptomatic after 26 months follow-up only receiving hydroxychloroquine 200 mg/day and belimumab |
| 2/female/36 | Erythematous papules and plaques on trunk Severe chilblain lupus in hands and feet |
Photosensitivity Lymphopenia Positive antinuclear antibodies |
Prednisone up to 60 mg/day for 11 years concomitantly with all the others treatments. Chloroquine 500 mg/day for 9 years concomitantly with the others treatments. Hydroxychloroquine 400 mg/day for 2 years sequentially to chloroquine. Methotrexate up to 20 mg/week for 4 years concomitantly with prednisone, chloroquine and isotretinoin. Isotretinoin 40 mg/day for 9 month concomitantly with prednisone, chloroquine and methotrexate. Mycophenolic acid up to 720 mg twice a day for 2 years sequentially to methotrexate. Azathioprine 200 mg/day for 2 years sequentially to mycophenolic acid. |
16: Red erythema on V-area neck (2), posterior neck and shoulders (2), chest (2), abdomen (2), rest of the face (2); and dark red erythema on hands (3) and feet (3) | 2: Pink erythema on V-area neck (1) and hands (1). | Total remission of subacute cutaneous lupus erythematosus lesions with mild chilblain lupus after 22 months follow-up receiving prednisone 5 mg/day and belimumab |
The second case was a woman in her ‘30s with a 11-year history of subacute cutaneous lupus erythematosus lesions (biopsy-proven) over the back and painful chilblains in her hands and feet. Blood tests revealed lymphopenia, positive antinuclear antibodies (1:640, homogeneous pattern), anti-Ro/SSA and anti-La/SSB antibodies. Remaining antibodies (anti-double stranded DNA, anti-Sm, anti-U1-RNP) were repeatedly negative. Serum biochemical parameters, acute phase reactants and complement levels (C3, C4 and CH50) were normal. The patient only fulfilled three out of four ACR criteria for systemic lupus erythematosus: lymphopenia, positive antinuclear antibodies and photosensitivity. Multiple systemic agents (including chloroquine 500 mg/day, hydroxychloroquine 400 mg/day, prednisone up to 60 mg/day, isotretinoin 40 mg/day, methotrexate 20 mg/week, mycophenolic acid 720 mg twice a day and azathioprine 200 mg/day) had been tried without clinical improvement [Figure 2a]. In addition, she developed severe adverse effects to treatment such as osteopenia as well as trigeminal and thoracic herpes zoster [Table 1]. Before starting belimumab, she was receiving prednisone 15 mg/day, hydroxychloroquine 400 mg/day and azathioprine 200 mg/day with a CLASI activity score of 16.
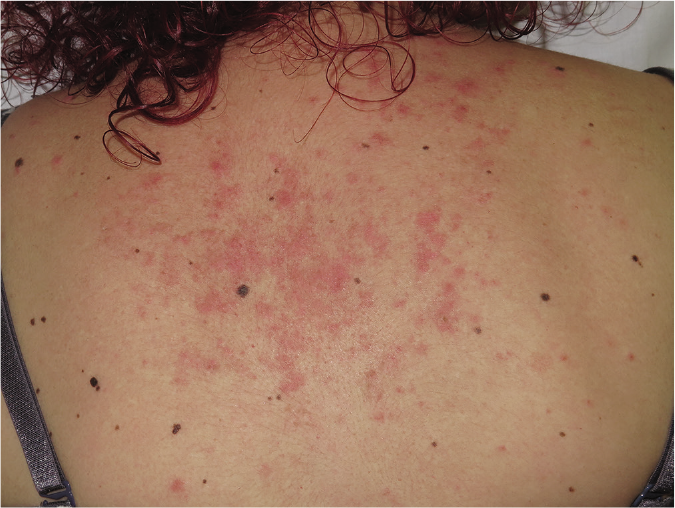
- Patient 2: Erythematous papules and plaques of subacute cutaneous lupus erythematosus on the back
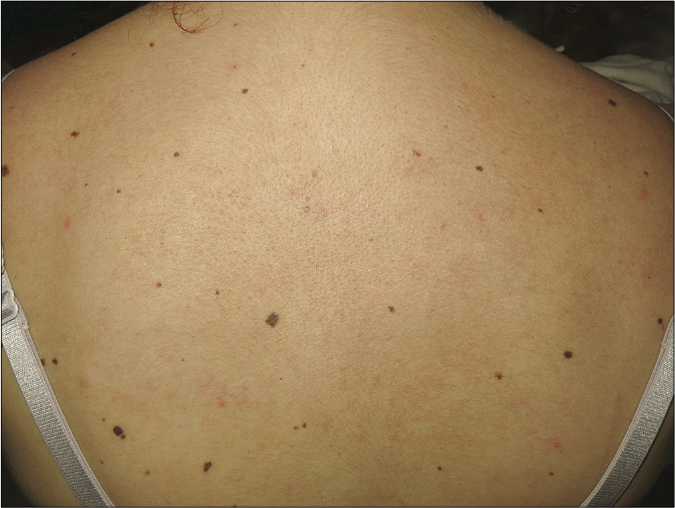
- Patient 2: Resolution of cutaneous lesions with belimumab therapy (6 months after initiation)
Owing to the difficulties in the management of both cases (refractory to high-doses of prednisone and multiple immunosuppressants and the appearance of adverse effects to medication), off-label treatment with belimumab, a drug with good efficacy reported in systemic lupus erythematosus, was requested and later approved by the therapeutic committee of our institution. The dosing was the same as the usual regimen used in systemic lupus erythematosus - 10 mg/kg IV on days 0, 14 and 28, and then every four weeks. The first patient experienced a complete resolution of cutaneous lupus erythematosus lesions in the initial three months itself [Figure 1b]. He remains asymptomatic with a CLASI score of 0 at 26-months follow-up, only receiving hydroxychloroquine 200 mg/day (prednisone and mycophenolic acid were discontinued after 8 and 23 months, respectively). In the second patient, subacute cutaneous lupus erythematosus lesions healed completely [Figure 2b], but mild chilblain lupus had persisted even after 22 months of treatment (CLASI score of 2). Azathioprine was stopped ten months after initiating belimumab and prednisone was tapered to 5 mg/day. At the moment, we want to continue the treatment indefinitely to avoid new flares.
Belimumab is a specific inhibitor of the B-lymphocyte survival stimulator (BLyS) that reduces auto-reactive B lymphocytes.2 Expression of BLyS is increased in cutaneous lupus erythematosus lesions,3 and belimumab therapy has been shown to cause marked improvement in the refractory cutaneous lesions in patients with systemic lupus erythematosus within 8-12 weeks.4 In a post-hoc analysis from two phase III trials, belimumab plus standard therapy has showed a significant reduction in mucocutaneous manifestations (rash, mucosal ulcers and alopecia) in systemic lupus erythematosus patients.5 In a recent study of antimalarial-refractory cutaneous lupus erythematosus, belimumab led to a 56% improvement in clinical response.1 Belimumab is generally well-tolerated, though infections, arthralgia, headache, rash, diarrhoea, nausea, infusion reactions, neutropenia and thrombocytopenia have been reported. Serious adverse events are rare, even though suicides due to severe depression and serious infections have been reported during trials.2 The patient’s condition must be regularly evaluated and discontinuation of belimumab considered if there is no clinical improvement after 6 months of treatment. Limited evidence is available regarding belimumab safety in pregnancy. Therefore, alternative medications should be considered for cutaneous lupus erythematosus treatment in this setting. Moreover, there is no data regarding the concentration of belimumab in breast milk and is better avoided in lactating patients. There are also no reports of belimumab use in children.6
Our report suggests that belimumab may be a good therapeutic alternative in refractory cases of cutaneous lupus erythematosus, even if they are not associated with systemic lupus erythematosus and also in those patients who develop severe adverse effects to standard therapy. Belimumab can be considered in the role of a long-term steroid-sparing drug.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Characteristics and alternative treatment outcomes of antimalarial refractory cutaneous lupus erythematosus. JAMA Dermatol. 2017;153:937-9.
- [CrossRef] [PubMed] [Google Scholar]
- Belimumab: First targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2:317-9.
- [CrossRef] [PubMed] [Google Scholar]
- High expression of B lymphocyte stimulator in lesional keratinocytes of patients with cutaneous lupus erythematosus. Exp Dermatol. 2018;27:95-7.
- [CrossRef] [PubMed] [Google Scholar]
- Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2017;26:857-64.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of belimumab, a B lymphocyte stimulator specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71:1833-8.
- [CrossRef] [PubMed] [Google Scholar]
- The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- [CrossRef] [PubMed] [Google Scholar]





