Translate this page into:
Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study
Correspondence Address:
Sujit J.S Shanshanwal
OPD No. 16, Department of Dermatology, LTMMC and Lokmanya Tilak Municipal General Hospital, Dr. Babasaheb Ambedkar Road, Sion West, Mumbai - 400 022, Maharashtra
India
| How to cite this article: Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol 2017;83:47-54 |
Abstract
Background: Finasteride and dutasteride are inhibitors of the enzyme 5-alpha-reductase which inhibits the conversion of testosterone to dihydrotestosterone. Dutasteride inhibits both type I and type II 5-alpha-reductase while finasteride inhibits only the type II enzyme. As both isoenzymes are present in hair follicles, it is likely that dutasteride is more effective than finasteride. Aims: To compare the efficacy, safety and tolerability of dutasteride and finasteride in men with androgenetic alopecia. Methods: Men with androgenetic alopecia between 18 and 40 years of age were randomized to receive 0.5 mg dutasteride or 1 mg finasteride daily for 24 weeks. The primary efficacy variables were hair counts (thick and thin) in the target area from modified phototrichograms and global photography evaluation by blinded and non-blinded investigators. The secondary efficacy variable was subjective assessment using a preset questionnaire. Patients were assessed monthly for side effects. Results: Ninety men with androgenetic alopecia were recruited. The increase in total hair count per cm[2] representing new growth was significantly higher in dutasteride group (baseline- 223 hair; at 24 weeks- 246 hair) compared to finasteride group (baseline- 227 hair; at 24 weeks- 231 hair). The decrease in thin hair count per cm[2] suggestive of reversal of miniaturization was significantly higher in dutasteride group (baseline- 65 hair; at 24 weeks- 57 hair) compared to finasteride group (baseline- 67 hair; at 24 weeks- 66 hair). Both the groups showed a similar side effect profile with sexual dysfunction being the most common and reversible side effect. Limitations: Limitations include the short duration of the study (6 months), the small sample size and the fact that it was an open-label study. Conclusions: Dutasteride was shown to be more efficacious than finasteride and the side-effect profiles were comparable.Introduction
Androgenetic alopecia is a common, genetically determined disorder affecting both men and women characterized by the gradual conversion of terminal hairs into indeterminate, and finally into vellus hairs.
Oral finasteride (1mg/day) has been approved by the United States of America Food and Drug Administration since December 1997 for the treatment of AGA in males. Finasteride is a type II 5-alpha reductase (5AR) inhibitor (5ARI), which significantly improves hair growth,[1] slows hair loss when compared with placebo,[2],[3],[4] and is a very commonly used treatment for AGA. In a study by Jung et al,[5] vertex hair in men with AGA presented improvement in only 48% of patients after 1 year and in only 66% at the end of 2 years when treated with finasteride 1 mg/day. Reviewing various studies reported in literature about the efficacy of finasteride in AGA, upto 30-50% of patients failed to show clinical improvement.[6],[7],[8],[9],[10],[11] Even though no more hair loss is also the therapeutic effect of finasteride, the patient's expectation from the treatment is not just arrest of the progression of AGA but also its reversal and improvement in their hair density and thickness. This prompts the need for alternate treatment modality. 5AR converts testosterone to dihydrotestosterone (DHT),[12] which is the principal androgen involved in the pathogenesis of AGA in men.[13] 5AR exists as 3 isoenzymes: types I, II and III.[14],[15],[16] Type I isoenzyme is mainly present in the skin, including the hair follicle and sebaceous glands,[17],[18] whereas type II is predominantly found in the male genitalia, including the prostate, but is also found in the inner root sheath of hair follicles.[12] Dutasteride, which is approved for symptomatic benign prostatic hyperplasia (BPH) at the daily dose of 0.5 mg, inhibits both type I and type II isoenzymes of 5AR. Dutasteride is approximately three times more potent than finasteride in inhibiting type I 5AR, and 100 times more potent in inhibiting type II 5AR.[19] Dutasteride at dose of 0.5 mg/day has shown to reduce serum DHT levels by more than 90%, while finasteride at a dose of 5 mg/day decreases serum DHT by 70%.[19],[20] Thus, theoretically dutasteride would be expected to have superior efficacy to finasteride for treating male with AGA. However, there is paucity of published data proving the same.
In androgenetic alopecia (AGA), gradual miniaturization of hair follicles androgenetic alopecia as well as hair shedding contribute to the appearance of hair loss, but which of these plays a major role is unclear.
Methods
Study participants
Sample size was calculated using the formula (Zα + Zβ) X ([σ1+ σ2)/(μ1− μ2]), where Zα =1.96, Zβ = 0.84, σ1 = standard deviation of one group (219.84), σ2 = standard deviation of second group (262.86), μ1 = mean of one group (927.5) and μ2 = mean of second group (902.1). Mean and standard deviation of the two groups were used from the study by Olsen et al.[21] The size was calculated as 31.17 for each group. To account for dropouts during the study, we selected a sample size of 45 in each group.[21]
Eligible men aged 18–40 years with androgenetic alopecia classified as Grade III vertex, IV or V (excluding types IV and V anterior) using the Hamilton Norwood classification were selected for the study. Patients having vertex involvement with female pattern hair loss not classifiable using the Hamilton Norwood classification were categorized as Grades F1, F2 and F3 using the basic and specific (BASP) classification. Patients using minoxidil, anti-androgenic medications or drugs causing hypertrichosis (phenytoin, acetazolamide, cyclosporine, diazoxide, psoralens, penicillamine, streptomycin and cortisone) or hypotrichosis (anti-coagulants, retinoids, lithium and beta blockers) within the past 6 months were excluded from the study. Patients with known systemic illnesses, smokers or tobacco chewers, or a history of breast or prostate cancer, or a first degree relative with prostate cancer before the age of 50 were also excluded.
The protocol was approved by the Institutional Ethics Committee.
Study design
A 24-week prospective, parallel, randomized, open-labeled and superiority study was conducted at the Department Of Dermatology, Lokmanya Tilak Municipal Medical College and Hospital, Sion, Mumbai from January 2013 to May 2014. Ninety patients who fulfilled the inclusion and exclusion criteria were recruited after taking informed consent and randomized to receive either 0.5 mg dutasteride or 1 mg finasteride daily for a period of 24 weeks. Patients were counseled by the primary investigator regarding the side effects of the medications prior to enrollment in the study. Patients were randomized using a randomized list generated from the “original generator” at www.randomization.com. The primary investigator possessed the randomization list and dispensed the medication without concealment of allocation.
Efficacy assessments
Global photography assessments
Clinical assessment was performed by blinded and non-blinded dermatologists using standardized photographs of the vertex scalp taken by placing the head on a stereotactic positioning device. They independently reviewed the paired photographs for hair growth taken at baseline and 24 weeks using a 7-point scale: Greatly increased (+3), moderately increased (+2), slightly increased (+1), unchanged (0), slightly decreased (−1), moderately decreased (−2) and greatly decreased (−3). This technique has been demonstrated to have excellent reproducibility.[6]
Phototrichogram assessments
Growth of new hair and reversal of miniaturization was estimated by total and thin hair counts performed in a target area of 1 cm [2] on the vertex bald spot with the hair clipped to a length of about 1 mm. Tattoos were diagonally placed to enhance reproducibility of the target area. A Heine ® Delta 20 dermatoscope attached to an adapter mounted on a Nikon ® D7000 single-lens reflex camera was used to capture magnified images of the target area to avoid missing any fine hair. Modified phototrichogram images of baseline and 24-week evaluation were printed on A3 size Kodak matt-photography paper. Changes in thick and thin hair counts were measured.
Subjective evaluation
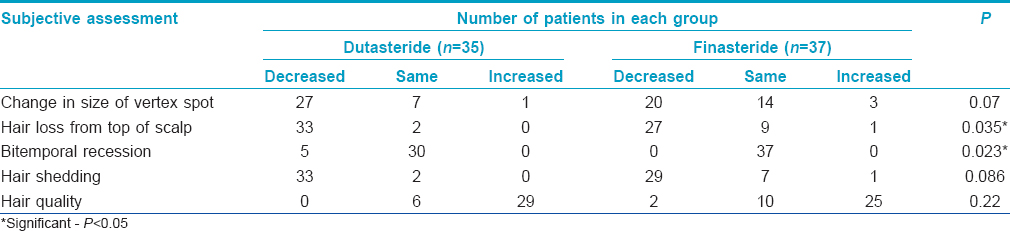
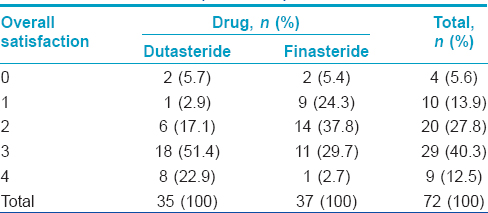
Subjects were asked to rate changes in the size of the vertex spot, hair loss on top of the scalp, bitemporal recession, the amount of hair shedding, hair quality and overall satisfaction with hair growth on a 3-point rating scale (increased, no change, or decreased). They were also asked to rate overall satisfaction on a scale of 0 (no change) to 5 (marked improvement).
Safety assessment
Safety assessment was performed through enquiry, physical examination and laboratory evaluation. Complete hemogram and liver function tests were conducted at baseline, 3 months and at completion of treatment (6 months). Sexual function was evaluated by specifically asking about the occurrence of decreased libido, erectile dysfunction and ejaculation disorders.
Statistical analysis
Data were analyzed using SPSS version 18 software by IBM Corporation. Chi-square test with continuity and Fischer's exact tests were used for evaluation of investigator assessment of global photographs and subjective parameters. Cohen's kappa (κ) was used to measure the degree of inter-investigator agreement. Mann–Whitney U-test was used to evaluate the hair count parameter and Fischer's exact test was used to compare adverse events, drug-related adverse events and adverse events leading to withdrawal from the study between treatment groups. P < 0.05 was considered statistically significant.
Results
A total of 72 patients completed the study. Reasons for dropping out in 18 patients included withdrawal of consent (4 patients) and sexual side effects (3 patients) while 11 patients were lost to follow-up. The dropout rate was comparable in both the groups (P = 0.793). Intention to treat analysis at 24 weeks revealed a significant increase in thick hair count in dutasteride group compared to the finasteride group (P = 0.030). A CONSORT flow diagram showing the progress through randomization in the two groups is depicted in [Figure - 1].
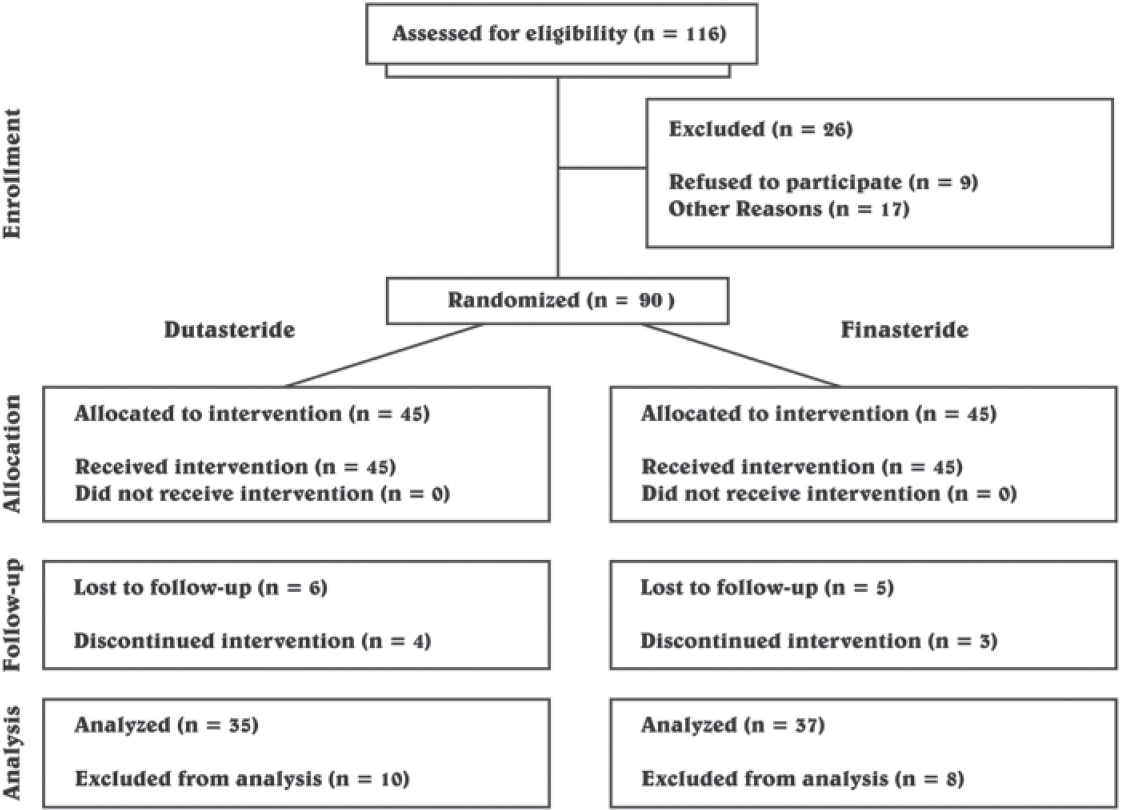 |
| Figure 1: CONSORT diagram |
Demography
The differences in the baseline characteristics of the dutasteride and finasteride groups were not statistically significant. The mean age of patients was 27.6 years in the dutasteride group and 28.1 years in the finasteride group. Patients with different grades of androgenetic alopecia were equally distributed in both the groups (P = 0.261). The most common grades of androgenetic alopecia in the study population were Grade IV (30.6% of patients) and Grade V (26.4% of patients) (P > 0.1).
Hair growth
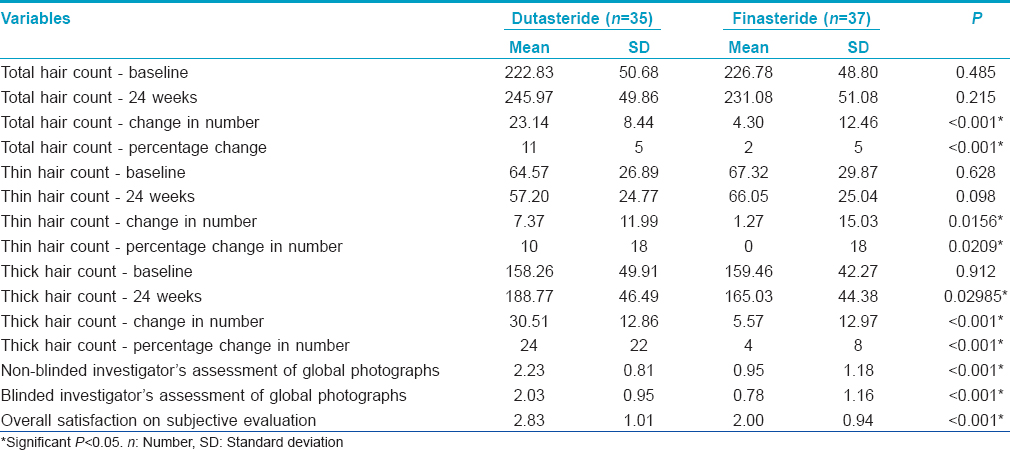
The mean change in total hair count after 24 weeks of treatment was significantly greater in the dutasteride group (23.14/cm [2]) compared with the finasteride group (4.3/cm [2]) [Figure - 2] and [Table - 1].
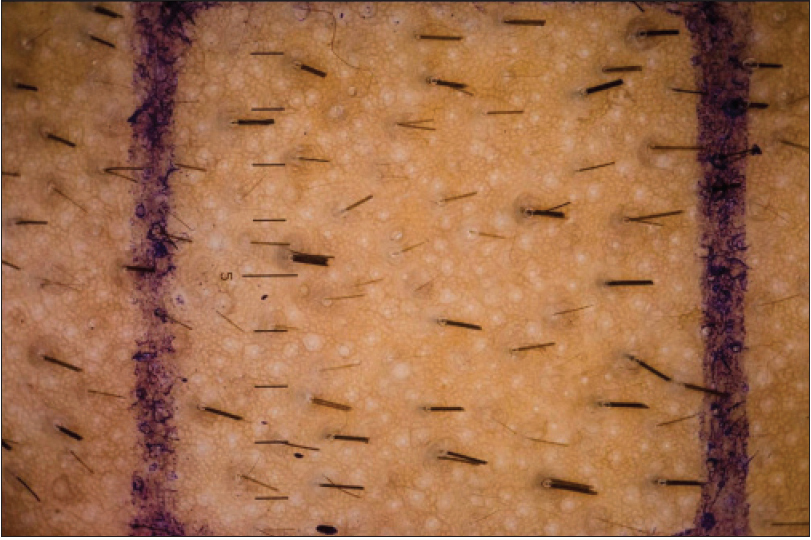 |
| Figure 2a: Pre treatment modified phototrichogram of patient on dutasteride |
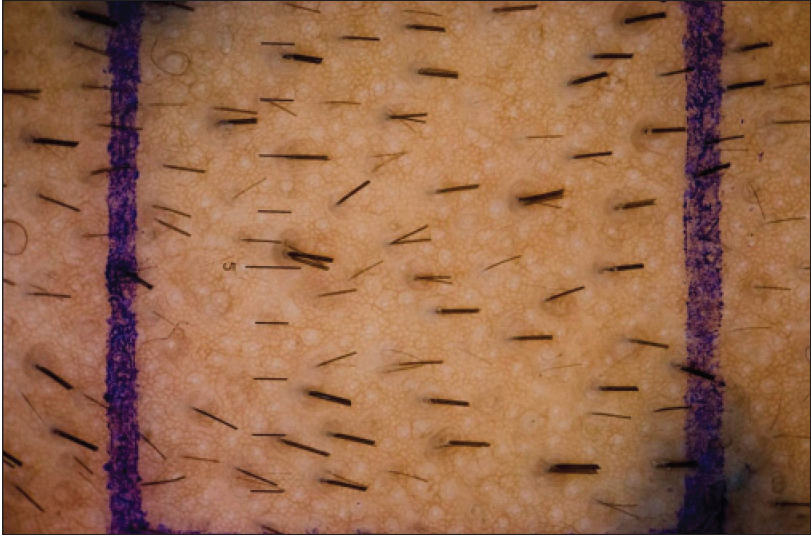 |
| Figure 2b: Post treatment modified phototrichogram of the same patient on dutasteride |
 |
| Figure 2c: Pre treatment modified phototrichogram of patient on finasteride |
 |
| Figure 2d: Post treatment modified phototrichogram of the same patient on finasteride |
Reversal of miniaturization
Thin hair counts decreased significantly (7.37/cm [2]) in the dutasteride group at 24 weeks as compared to the finasteride group (1.27/cm [2], P = 0.0156) [Figure - 2] and [Table - 1]. The difference was statistically significant.
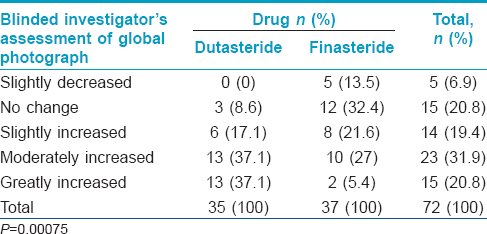
Global photography assessments
The proportion of patients with marked improvement was higher in the dutasteride group [Figure - 3] and [Table - 2] in both blinded and non-blinded evaluations (P < 0.001). There was substantial agreement in the individual scores assigned by the two dermatologists for assessment of hair growth (kappa coefficient 0.742 and 0.789 in the dutasteride and finasteride groups respectively).
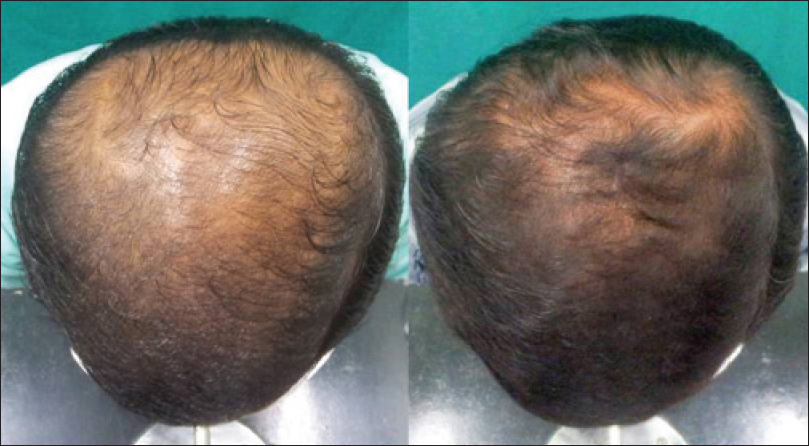 |
| Figure 3a: Pre and post treatment global photographs of great improvement in patient with androgenetic alopecia at week 24 on dutasteride |
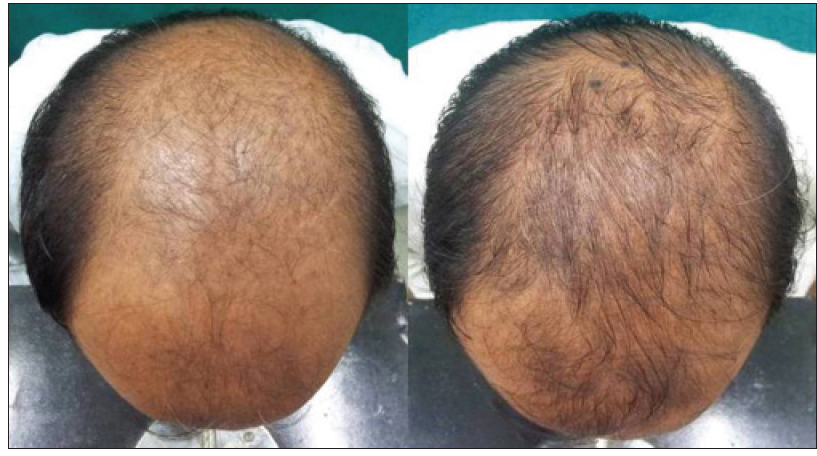 |
| Figure 3b: Pre and post treatment global photographs of great improvement in patient with androgenetic alopecia at week 24 on finasteride |
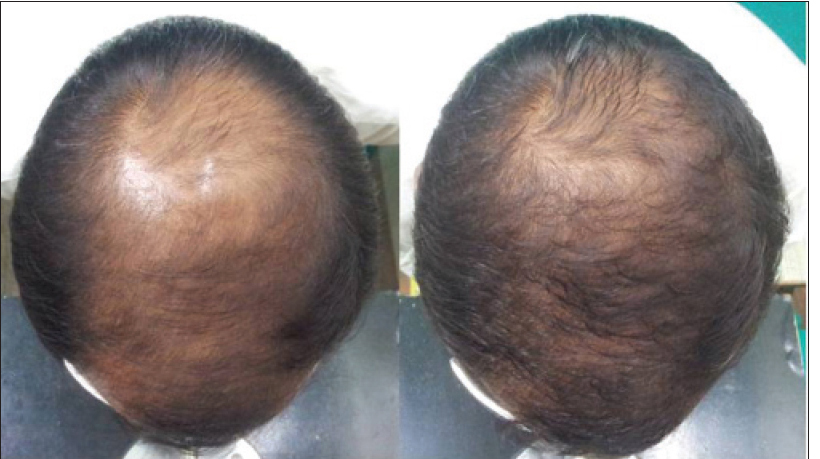 |
| Figure 3c: Pre and post treatment global photographs of moderate improvement in patient with androgenetic alopecia at week 24 on dutasteride |
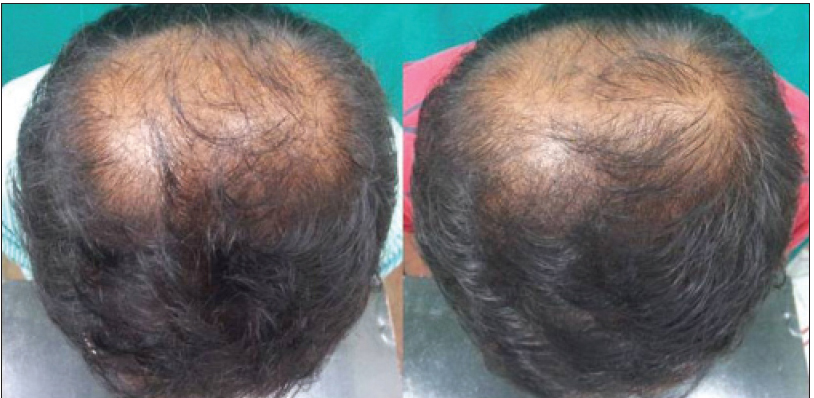 |
| Figure 3d: Pre and post treatment global photographs of slight improvement in patient with androgenetic alopecia at week 24 on finasteride |
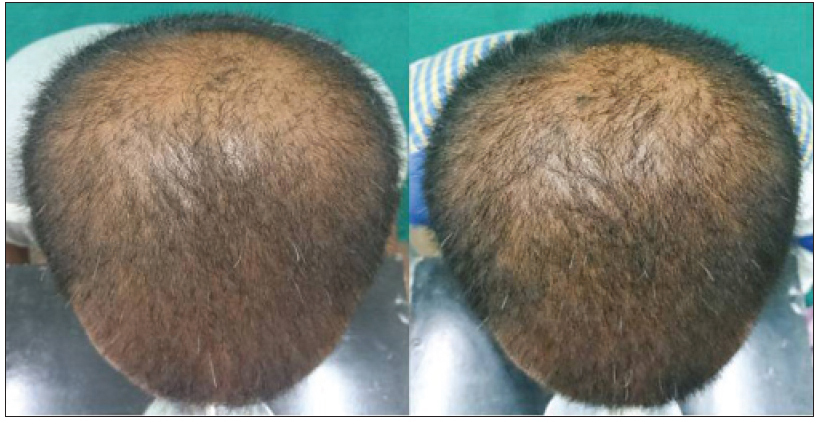 |
| Figure 3e: Pre and post treatment global photographs of slight improvement in patient with androgenetic alopecia at week 24 on finasteride |
Subjective evaluation
The mean change from baseline in the subjective evaluation of hair growth scale (loss of hair from the top of scalp, recession of temporal hairline and overall satisfaction) was significantly better in the dutasteride group compared to finasteride group (P < 0.05). However, there was no significant difference in terms of size of vertex balding spot, hair shedding and quality of hair between the two groups [Table - 3] and [Table - 4].
Safety
Most adverse events reported post-randomization were mild to moderate (dutasteride group, n = 8; finasteride group, n = 7). The overall incidence of adverse events was similar in the two treatment groups. There was no statistical difference between dutasteride and finasteride in the incidence of adverse events, drug-related adverse events or adverse events leading to withdrawal from the study. Erectile dysfunction and loss of libido was seen in 3 and 4 patients in the dutasteride group as compared to 1 and 3 patients in finasteride group, respectively but this was not statistically significant.
Discussion
In our study, dutasteride 0.5 mg was found to be significantly more effective than finasteride 1 mg in men aged 18–40 years with androgenetic alopecia as assessed by hair counts, subject self-assessment and blinded and non-blinded evaluation of global photographs.
In a 24-week, double-blind, placebo controlled, dose-ranging study by Olsen et al., dutasteride increased hair growth in a dose-dependent manner. Dutasteride, 0.5 mg and 2.5 mg significantly improved hair counts after 24 weeks as compared to finasteride, 5 mg.[21] Other studies have confirmed efficacy of oral dutasteride compared with placebo in men with androgenetic alopecia [22],[23],[24] as well as in comparison with finasteride.[5],[22] Jung et al. treated 31 Korean men with androgenetic alopecia who had not shown significant improvement when treated with finasteride 1 mg for at least 6 months with dutasteride 0.5 mg. After 6 months, the hair density and thickness increased by 10.3% and 18.9% respectively.[5]
The change in hair counts in the dutasteride group in our study was comparable with previously published data.[21],[22],[23] However, change in hair counts in the finasteride group was seen to be lower in contrast to other studies.[21],[22],[23] This may be related to steroid-5-alpha-reductase alpha polypeptide 2 gene polymorphism. The V89L site leucine substitution of valine at codon 89 polymorphism reduces in vivo 5-alpha reductase activity.[25] The V89L site has been shown to be highly polymorphic in the Indian population.[26] The increase in the number of thin hair at the end of treatment in the finasteride group suggests a failure of finasteride in reversing the miniaturization process.
The incidence of sexual side effects in this study in the dutasteride group (15.6%) was similar to that reported by Jung et al. (17.1%).[5] Dutasteride-related sexual dysfunction had been shown to decrease in frequency when treatment was continued for up to 4 years.[27],[28] In this study, dutasteride and finasteride were relatively well-tolerated with comparable sexual side effects in both the groups which were consistent with previously reported data.[21],[22],[23] Although persistent sexual side effects associated with finasteride have been publicized in the lay press, controlled clinical data show a low incidence of sexual side effects that resolve on cessation of treatment.[29] Several large population-based long-term placebo-controlled studies have demonstrated no clear evidence of the negative effect of 5-alpha reductase inhibitor on erectile function.[30] Erectile dysfunction has also been reported to be a nocebo effect.[31]
The limitations of this study include the short (6 months) duration of the study, the small sample size and the fact that it was an open-label study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia. J Dermatol 2012;39:27-32.
[Google Scholar]
|
| 2. |
Kawashima M, Hayashi N, Igarashi A, Kitahara H, Maeguchi M, Mizuno A, et al. Finasteride in the treatment of Japanese men with male pattern hair loss. Eur J Dermatol 2004;14:247-54.
[Google Scholar]
|
| 3. |
Lin JH, Chen WC. Finasteride in the treatment of Taiwanese men with androgenetic alopecia: A 12-month open-label study. Kaohsiung J Med Sci 2002;18:379-85.
[Google Scholar]
|
| 4. |
Finasteride Male Pattern Hair Loss Study Group. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol 2002;12:38-49.
[Google Scholar]
|
| 5. |
Jung JY, Yeon JH, Choi JW, Kwon SH, Kim BJ, Youn SW, et al. Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol 2014;53:1351-7.
[Google Scholar]
|
| 6. |
Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride male pattern hair loss study group. J Am Acad Dermatol 1998;39(4 Pt 1):578-89.
[Google Scholar]
|
| 7. |
McClellan KJ, Markham A. Finasteride: A review of its use in male pattern hair loss. Drugs 1999;57:111-26.
[Google Scholar]
|
| 8. |
Van Neste D, Fuh V, Sanchez-Pedreno P, Lopez-Bran E, Wolff H, Whiting D, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol 2000;143:804-10.
[Google Scholar]
|
| 9. |
Price VH, Menefee E, Sanchez M, Kaufman KD. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): Three- and 4-year results. J Am Acad Dermatol 2006;55:71-4.
[Google Scholar]
|
| 10. |
Roberts JL, Fiedler V, Imperato-McGinley J, Whiting D, Olsen E, Shupack J, et al. Clinical dose ranging studies with finasteride, a type 2 5alpha-reductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol 1999;41:555-63.
[Google Scholar]
|
| 11. |
Whiting DA, Olsen EA, Savin R, Halper L, Rodgers A, Wang L, et al. Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur J Dermatol 2003;13:150-60.
[Google Scholar]
|
| 12. |
Chen W, Zouboulis CC, Orfanos CE. The 5 alpha-reductase system and its inhibitors. Recent development and its perspective in treating androgen-dependent skin disorders. Dermatology 1996;193:177-84.
[Google Scholar]
|
| 13. |
Messenger A. Male androgenetic alopecia. In: Blume-Peytavi U, Tosti A, Whiting DA, Trueb R, editors. Hair Growth and Disorders. Berlin: Springer; 2008. p. 159-70.
[Google Scholar]
|
| 14. |
Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin 1996;14:697-711.
[Google Scholar]
|
| 15. |
Olsen EA, editor. Disorders of Hair Growth: Diagnosis and Treatment. New York: McGraw-Hill; 1994. p. 257-83.
[Google Scholar]
|
| 16. |
Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, et al. 5a-reductase type 3 expression in human benign and malignant tissues: A comparative analysis during prostate cancer progression. Prostate 2011;71:1033-46.
[Google Scholar]
|
| 17. |
Sato T, Sonoda T, Itami S, Takayasu S. Predominance of type I 5alpha-reductase in apocrine sweat glands of patients with excessive or abnormal odour derived from apocrine sweat (osmidrosis). Br J Dermatol 1998;139:806-10.
[Google Scholar]
|
| 18. |
Thiboutot D, Harris G, Iles V, Cimis G, Gilliland K, Hagari S. Activity of the type 1 5 alpha-reductase exhibits regional differences in isolated sebaceous glands and whole skin. J Invest Dermatol 1995;105:209-14.
[Google Scholar]
|
| 19. |
Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004;89:2179-84.
[Google Scholar]
|
| 20. |
Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab 1994;79:703-6.
[Google Scholar]
|
| 21. |
Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol 2006;55:1014-23.
[Google Scholar]
|
| 22. |
Gubelin Harcha W, Barboza Marttza J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol 2014;70:489-498.e3.
[Google Scholar]
|
| 23. |
Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol 2010;63:252-8.
[Google Scholar]
|
| 24. |
Stough D. Dutasteride improves male pattern hair loss in a randomized study in identical twins. J Cosmet Dermatol 2007;6:9-13.
[Google Scholar]
|
| 25. |
Makridakis N, Ross RK, Pike MC, Chang L, Stanczyk FZ, Kolonel LN, et al. A prevalent missense substitution that modulates activity of prostatic steroid 5alpha-reductase. Cancer Res 1997;57:1020-2.
[Google Scholar]
|
| 26. |
Rajender S, Vijayalakshmi K, Pooja S, Madhavi S, Paul SF, Vettriselvi V, et al. Longer (TA) n repeat but not A49T and V89L polymorphisms in SRD5A2 gene may confer prostate cancer risk in South Indian men. J Androl 2009;30:703-10.
[Google Scholar]
|
| 27. |
Roehrborn CG, Marks LS, Fenter T, Freedman S, Tuttle J, Gittleman M, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology 2004;63:709-15.
[Google Scholar]
|
| 28. |
Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C; ARIA, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol 2004;46:488-94.
[Google Scholar]
|
| 29. |
Gupta AK, Charrette A. The efficacy and safety of 5a-reductase inhibitors in androgenetic alopecia: A network meta-analysis and benefit-risk assessment of finasteride and dutasteride. J Dermatolog Treat 2014;25:156-61.
[Google Scholar]
|
| 30. |
Anitha B, Inamadar AC, Ragunatha S. Finasteride-its impact on sexual function and prostate cancer. J Cutan Aesthet Surg 2009;2:12-6.
[Google Scholar]
|
| 31. |
Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, et al. Finasteride 5 mg and sexual side effects: How many of these are related to a nocebo phenomenon? J Sex Med 2007;4:1708-12.
[Google Scholar]
|
Fulltext Views
49,581
PDF downloads
6,258





