Translate this page into:
Suppression of oxidative-stress induced melanocyte death: Role of poly(ADP-ribose) polymerase in vitiligo pathogenesis
Corresponding author: Prof. Rasheedunnisa Begum, Department of Biochemistry, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat, India. rasheedunnisab@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Singh M, Mansuri MS, Dwivedi M, Kadam A, Mayatra JM, Laddha N, et al. Suppression of oxidative-stress induced melanocyte death: Role of poly(ADP-ribose) polymerase in vitiligo pathogenesis. Indian J Dermatol Venereol Leprol 2022;88:413-5.
Sir,
Vitiligo is an acquired depigmentation disorder linked with genetic and non-genetic etiologic factors. Oxidative stress has been implicated as the initial triggering factor for melanocyte destruction in vitiligo.1 The poly adenosine diphosphateribose polymerases (PARPs) are a group of nuclear enzymes that repair DNA damage by polyADP-ribosylation using NAD+ as a substrate and implicated in vital cellular processes. Oxidative stress triggers DNA damage and hyperactivation of PARP1s, leading to cell dysfunction and death.2 The present study aimed to assess the impact of inhibition of PARPs using 1,5-dihydroxyisoquinoline on hydrogen peroxide (H2O2) induced oxidative stress in in vitro cultured normal human melanocytes.
The study plan was approved by the university’s Institutional Ethics Committee for Human Research (FS/IECHR/BC/RB/1). The skin samples were procured by punch biopsy and/or from the left-over skin after surgery to be used for melanocyte culturing. The rescue from oxidative stress in H2O2 treated normal human melanocytes was studied by inhibition of PARPs using 1,5-dihydroxyisoquinoline. Cell viability was monitored by trypan blue exclusion assay; cleavage and activity of PARPs were monitored by Western blotting and the transcript levels were estimated by real-time polymerase chain reaction. For in vitro studies, triplicate experiments’ data was analysed by Student’s t-test and oneway analysis of variance (Prism 6, GraphPad Software, Inc; San Diego, CA) as mean±standard error of the mean.
Our study observed a dose-dependent decrease in cell viability on H2O2 treatment, that is, 100 µM (P = 0.0248), 250 µM (P = 0.0014) and 500 µM (P < 0.0001) [Figure 1b]. 100 µM H2O2 (Lethal concentration 50% dose) was selected for downstream experiments. 1,5-dihydroxyisoquinoline (50 µM, 100 µM and 200 µM) could not significantly affect the morphology and viability of normal human melanocytes [Figure 1c]. Further, we monitored rescue of normal human melanocytes death with and without oxidant treatment by 1,5-dihydroxyisoquinoline. Interestingly, 100 µM 1,5-dihydroxyisoquinoline pre-treated cells showed a significant rescue from the cytotoxic effects of H2O2 on cell morphology [Figure 1a] and viability [Figure 1d]. A significant rescue was observed at 250 µM (P = 0.0022) and 500 µM (P = 0.0002) H2O2. However, no significant rescue was seen at 100 µM H2O2 (P = 0.0471) [Figure 1d].

- Morphological effect of 1,5-dihydroxyisoquinoline on normal human melanocytes: No significant morphological effect was observed on 1,5-dihydroxyisoquinoline exposure on normal human melanocytes morphology. A significant rescue was observed from a dose-dependent H2O2 mediated cell death on 1,5-dihydroxyisoquinoline pre-treatment. Magnification ×10
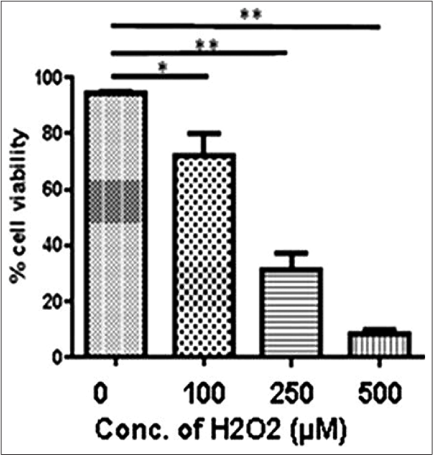
- A significant cell death was seen at 100 µM, 250 µM and 500 µM H2O2, respectively, (P = 0.0248, P = 0.0027 and P = 0.0021) compared to control
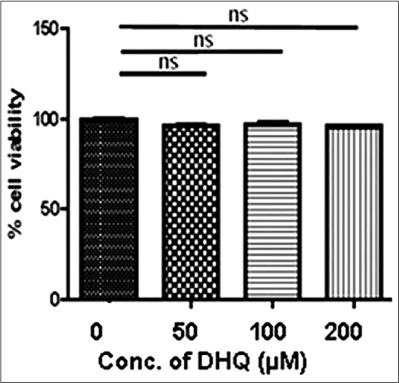
- No significant effect on normal human melanocytes viability was observed on 1,5-dihydroxyisoquinoline exposure
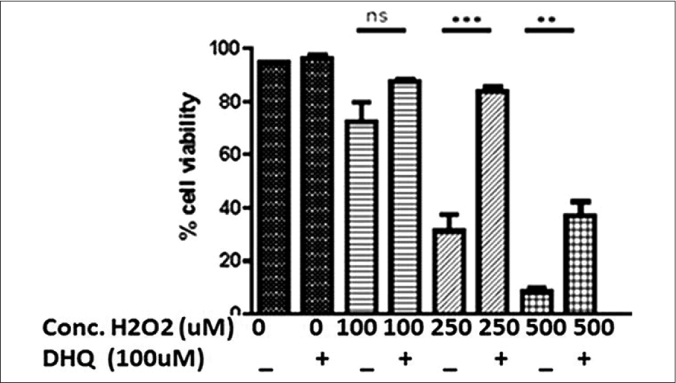
- A dose-dependent effect of H2O2 on normal human melanocytes viability with and without pre-treatment (4 hours) of 100 µM 1,5-dihydroxyisoquinoline. Significant rescue in cell death was observed at higher doses of H2O2 (P = 0.0471; P = 0.0002 and P = 0.0022); the values represent mean ± standard deviation of three independent experiments
Earlier reports suggested the activation of caspases 3, 8 and 9 and elevated cleavage of PARPs in the depigmented epidermis compared with the normally pigmented one. Tulic group3 also observed increased activation of p38 and poly (ADP-ribose) polymerases cleavage in vitiligo patients. We further studied PARylation and PARP-1 activation on 1,5-dihydroxyisoquinoline mediated rescue from H2O2 induced apoptosis. 1,5-dihydroxyisoquinoline exhibited a significant restoration of a few normal human melanocytes proteins which were affected by H2O2. Hyperactivation of PARP-1 led to PARP-1 cleavage resulting in release of 89 kDa fragment followed by apoptosis. On the contrary, 1,5-dihydroxyisoquinoline suppressed the PARP-1 cleavage and apoptosis [Figure 2b]. Furthermore, normal human melanocytes exhibited a significant restoration in polyADP-ribosylation pattern and PARP-1 activation in 1,5-dihydroxyisoquinoline+H2O2 treated group compared to only H2O2 treated group [Figures 2a and 2b]. In line with our observations, the previous studies showed 1,5-dihydroxyisoquinoline rescued human cardiac myoblasts and rat cardiomyocytes exposed to H2O2. It has also been shown that 1,5-dihydroxyisoquinoline inhibit PARP activity and protect endothelial cells from oxidative stress.4 The effect of oxidative stress on microphthalmia-associated transcription factor-M (MITF-M), tyrosinase and intercellular adhesion molecule-1 (ICAM-1) expression was also studied in 50 µM and 100 µM of H2O2 treated normal human melanocytes. We observed, significantly decreased MITF-M transcript (P = 0.0083 and P = 0.0383) and protein (P = 0.0024 and P < 0.0001) levels at both H2O2 doses, respectively, [Figures 3a, 3d and 3e]. MITF regulates the expression of melanocyte-specific proteins required for melanin synthesis. Tyrosinase, the target gene for MITF-M was decreased in 50 µM (P = 0.0109) and 100 µM (P = 0.0439) H2O2 treated cells [Figure 3b]. However, there was no significant difference found in ICAM-1 transcript post-treatment with 50 µM (P = 0.0772) and 100 µM H2O2 (P = 0.1325) [Figure 3c]. In a recent review,5 parthanatos, which is PARP-1 dependent cell death, has been suggested as instrumental in oxidative stress-related diseases such as vitiligo and hence, inhibiting parthanatos to make melanocyte step back from the brink of parthanatotic cell death might be well pursuing. However, the exact role of parthanatos in vitiligo pathogenesis through PARP-1 activation need to be be explored in vivo and ex vivo using animal and human skin models.
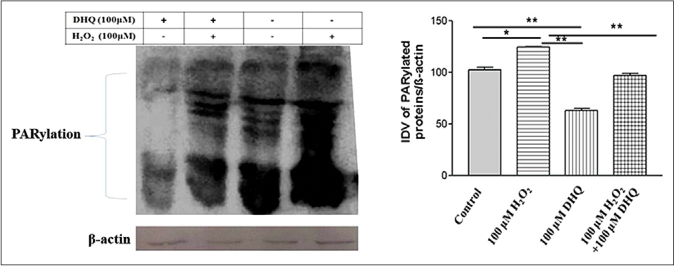
- Analysis of PARylation and poly (ADP-ribose) polymerase 1 activation on 1,5-dihydroxyisoquinoline mediated rescue from H2O2 induced cell death: Densitometric analysis for 1,5-dihydroxyisoquinoline and 1,5-dihydroxyisoquinoline + H2O2 groups revealed a significant difference (P = 0.0076) in PARylation suppression

- Analysis of PARylation and PARP-1 activation on 1,5-dihydroxyisoquinoline mediated rescue from H2O2 induced cell death: PARP-1 hyperactivation was observed in (H2O2) group (89 kDa cleaved fragment). Densitometric analysis for 1,5-dihydroxyisoquinoline alone group and 1,5-dihydroxyisoquinoline + H2O2 group also revealed a significant difference (P = 0.0062) in PARP-1 activation. Beta-actin: A protein loading control. Results are mean ± standard deviation of three independent experiments

- Effect of oxidative stress on microphthalmia-associated transcription factor-M, Tyrosinase and Intercellular Adhesion Molecule 1 and A dose-dependent effect of H2O2 on microphthalmia-associated transcription factor-M: (a) Microphthalmia-associated transcription factor-M transcript was significantly reduced in H2O2 treated cells compared to control (P = 0.0083 and P = 0.0383). (b) Tyrosinase transcript was also significantly reduced in H2O2 treated cells as compared to control (P = 0.0439 and P = 0.0109). (c) There was no significant difference in Intercellular Adhesion Molecule 1 transcript on H2O2 treatment compared to control (P = 0.0772 and P = 0.1325). Immunofluorescence analysis revealed a significant decrease in microphthalmia-associated transcription factor-M in normal human melanocytes treated with (d) 50 µM and (e) 100 µM of H2O2 for 24 h (P = 0.0024 and P = 0.0001). Results are mean ± standard deviation of three independent experiments (magnification ×63)
Collectively, this novel preliminary study supports that inhibition of poly (ADP-ribose) polymerases 1 by 1,5-dihydroxyisoquinoline attenuates H2O2 induced melanocyte death, signifying the role of PARP-1s in oxidative-stress mediated melanocyte death and in developing a potential therapeutic target for vitiligo.
Acknowledgment
RB thanks Indian Council of Medical Research (BMS/Ad hoc/122/11-2012) and the Department of Biotechnology (BT/PR9024/MED/12/332/2007), New Delhi, India. GS thanks Shastri Research Grant (SRG 2013–2014), Shastri Indo-Canadian Institute, Calgary, Canada.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
1) Indian Council of Medical Research (ICMR) (Ref. No. BMS/Ad hoc/122/11-2012), New Delhi, India. 2) Department of Biotechnology (DBT) (Ref. No. BT/ PR9024/MED/12/332/2007), New Delhi, India. 3) Shastri Research Grant (SRG 2013-2014), Shastri Indo-Canadian Institute, Calgary, Canada.
Conflicts of interest
There are no conflicts of interest.
References
- Role of oxidative stress and autoimmunity in onset and progression of vitiligo. Exp Dermatol. 2014;23:352-3.
- [CrossRef] [PubMed] [Google Scholar]
- PolyADP-ribose polymerase-1: Beyond transcription and towards differentiation. Semin Cell Dev Biol. 2017;63:167-79.
- [CrossRef] [PubMed] [Google Scholar]
- Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun. 2019;10:2178.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684-91.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of melanocyte death in vitiligo. Med Res Rev. 2021;41:1138-66.
- [CrossRef] [PubMed] [Google Scholar]





