Translate this page into:
Survival and prognostic factors in bullous pemphigoid: A retrospective cohort study
Corresponding author: Dr. Cristian Papara, Department of Dermatology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania. cristian.papara@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Papara C, Chiorean R, Leucuta D, Baican C, Danescu S, Sitaru C, et al. Survival and prognostic factors in bullous pemphigoid: A retrospective cohort study. Indian J Dermatol Venereol Leprol 2023;89:363-71.
Abstract
Background
Bullous pemphigoid is the most common subepidermal autoimmune blistering disease. Till now, the reported prognostic factors in bullous pemphigoid vary considerably.
Aims
The purpose of this study was to determine the overall survival rate and prognostic factors in bullous pemphigoid.
Methods
We conducted a retrospective cohort study on newly diagnosed bullous pemphigoid patients between July 2001 and November 2019 in a referral unit for autoimmune blistering skin diseases in Romania.
Results
One hundred forty-eight patients were included in the study. The Kaplan-Meier overall survival rates at 1, 3, 5 and 10 years were respectively 74.2% (95% confidence interval, 67.5-81.6%), 53.4% (45.7-62.2%), 43.6% (35.9-53%) and 31.3% (23.5-41.7%). The median follow-up among survivors was 48 months (interquartile range: 11-150). Ninety (60.8%) patients died during the follow-up period; of them, 38 (42.2%) had active disease at the time of death. Advanced age, neurological diseases, valvular heart disease, malignancies, use of statins, skin infections and extensive cutaneous involvement were linked to poorer outcomes, while the use of topical corticosteroids was associated with increased overall survival.
Limitations
This study lacks a control cohort to validate the obtained results. It was conducted in a retrospective manner in a single centre. In addition, indirect immunofluorescence microscopy was not performed in all patients.
Conclusion
Beyond ageing and neurological comorbidities, the prognosis of bullous pemphigoid patients was significantly influenced by the presence of skin infections, valvular heart disease, use of statins and extensive cutaneous involvement. Topical corticosteroid treatment was associated with increased survival in these patients.
Keywords
Bullous pemphigoid
epidemiology
prognostic factors
survival
topical corticosteroids
Plain Language Summary
Bullous pemphigoid is a skin disease where spontaneous blisters form due to abnormal antibodies produced by the patient’s own immune system (autoimmune disease). Since it is the most common autoimmune blistering skin disease, which is associated with high mortality and an increasing incidence, it is important to identify predictors for the survival of these patients. Our study has shown that advanced age, neurological diseases, disease of the heart valves, malignancies, use of statins (a group of blood cholesterol-lowering medications), skin infections at the time of diagnosis and widespread skin involvement are associated with lower survival rates. The use of corticosteroid creams/ ointments was linked to increased survival. These identified factors may help dermatologists in the reduction of disease mortality by identifying high-risk patients.
Introduction
Bullous pemphigoid is the most common subepidermal autoimmune blistering skin disease.1 Over the past two decades, a significant increase in bullous pemphigoid incidence has been observed. This is mainly attributed to an ageing population, certain culprit drugs, as well as enhanced diagnostic techniques.2
Old age, low Karnofsky scores, neurologic and cardiac diseases, prolonged hospitalisation, elevated levels of circulating anti-BP180 antibodies, hypoalbuminemia, polypharmacy and high-dose corticosteroids had all been linked to poor prognosis among bullous pemphigoid patients.3-10 Yet, many of these risk factors have remained understudied or have not been validated in larger cohorts. In addition, studies regarding the survival and prognostic factors of bullous pemphigoid in Romania have not been published so far.
We sought to evaluate the overall survival and to identify potential risk factors for lethal outcome in bullous pemphigoid patients in a representative cohort from a tertiary referral unit for autoimmune bullous diseases in Romania.
Methods
We performed an observational document-based retrospective study of patients diagnosed with bullous pemphigoid from July 2001 to November 2019 at the Department of Dermatology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania. This is the only centre in the North-West region of the country which provides an immunopathological diagnosis of autoimmune bullous diseases.
For inclusion, patients with a diagnosis of bullous pemphigoid had to fulfil all of the following three criteria:
Consistent clinical findings (generalized tense inflammatory blisters with or without persistent urticarial or eczematous lesions),
Direct immunofluorescence microscopy demonstrating linear deposition of immunoglobulin G and/or C3 along the basement membrane, and
-
Enzyme-linked immunosorbent assay with positive immunoglobulin G autoantibodies against BP180 and/or BP230, plus at least one of the following two additional criteria:
Histopathological evidence of a subepidermal blister/cleft with eosinophils, or
indirect immunofluorescence microscopy demonstrating circulating autoantibodies bound to the epidermal side of salt-split skin.
Patients with isolated or predominant oral disease, those with negative direct immunofluorescence microscopy and negative enzyme-linked immunosorbent assay autoantibodies were excluded from the study. For the detection of specific serum autoantibodies, commercially available enzyme-linked immunosorbent assay kits were used (MBL laboratories, Nagoya, Japan). The cut-off value for positive levels of anti-BP180 and anti-BP230 autoantibodies was ≥9 U/mL. Patients’ sera harvested at diagnosis were frozen and stored at -80 degrees Celsius. Between 2001 and 2008 enzyme-linked immunosorbent assay-based serum autoantibody detection was performed at the Department of Dermatology, University of Lübeck, Lübeck, Germany, and the Department of Dermatology, University of Würzburg, Würzburg, Germany. Since 2008, the enzyme-linked immunosorbent assay probes were analysed in the Department of Dermatology, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
The study was approved by the Ethics Committee of the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca (decision number 47/2020) and was conducted in accordance with the principles of the Declaration of Helsinki. All patients signed informed consent at initial consultation agreeing to be photographed for medical documentation, their clinical and laboratory data to be recorded, as well as blood and tissue samples to be used for scientific research.
Variables
For each patient included in the study, the following information was obtained from medical records: gender, age at diagnosis, date of diagnosis (the day of positive direct immunofluorescence microscopy diagnosis), diagnostic delay, current medications, clinical manifestations, distribution of cutaneous lesions, mucosal involvement, skin infections at diagnosis requiring systemic antibiotics, histo- and immunopathological features, biochemical and serological parameters, including bullous pemphigoid-related autoantibody serum titers, treatment of bullous pemphigoid (topical and systemic corticosteroids, other immunosuppressive drugs, antihistamines), follow-up period as well as date and cause of death. Local treatment included high-potency topical corticosteroids applied only on lesional skin. Telephone interviews with patients were performed in order to obtain the data missing from patients’ files. Death records data were obtained from the National Romanian Population Register.
Disease severity at diagnosis was assessed based on the percentage of body surface area involvement (mild <10%, moderate 10-30% and severe ≥30%), and the autoimmune bullous skin disorder intensity score (0-150). The score was calculated retrospectively for patients included in the study between 2001 and 2007 based on stored clinical photographs and medical records. After 2007, the autoimmune bullous skin disorder intensity score was directly performed in accordance with available recommendations.11
The following comorbidities were analysed during the whole follow-up period: cardiovascular (e.g., ischemic heart disease), neurological (e.g., stroke), pulmonary, renal and thyroid disease; diabetes and malignancy.
Endpoints
The endpoint of our study was the overall survival of bullous pemphigoid patients during their follow-up period, defined from the index date (the first appearance in the medical records), until the end of the study in February 2020 or until the time of demise. The study duration was 18 years, with a median follow-up period of 48 months.
Statistical analysis
Survival data was presented as survival probability at different points in time, and it was represented graphically with Kaplan-Meier plots. To identify prognostic factors for survival, we used the log-rank test and univariate Cox proportional hazard models. Next, we built multivariate Cox proportional hazard models using one variable of interest and adjusted for variables known to be linked with survival: age at onset (years), cutaneous involvement, autoimmune bullous skin disorder intensity score, anti-BP180 autoantibodies measured by enzyme-linked immunosorbent assay, prednisone dose (0.5 mg/kg/day) and immunosuppressive therapy. For all models, we checked the proportional hazard assumption with a formal test, and we presented for each variable of interest the hazard ratio and its 95% confidence interval. All multivariate models were checked for multi-collinearity using the variance inflation factor. The functional form was checked with partial residual plots.
For all statistical tests, a 0.05 confidence level was used, and the two-tailed P-value was computed. All statistical analyses were performed using the R environment for statistical computing and graphics, version 3.6.2. Statistical significance was defined as a P-value < 0.05.
Results
A total of 148 patients (54.1% females) met the enrolment criteria [Table 1]. The median age for bullous pemphigoid diagnosis was 74.5 years (range, 15-92 years). The median time from onset to diagnosis was 61.5 days (interquartile range, 31-151.3 days).
| Characteristic | Value |
|---|---|
| Total patients | 148 |
| Sex Male Female |
68 (46%) 80 (54%) |
| Age at diagnosis (years) <60 60-69 70-79 ≥80 |
20 (13.5%) 26 (17.6%) 54 (36.5%) 48 (32.4%) |
| Onset Blisters with pruritus Blisters without pruritus Non-bullous lesions with pruritus Non-bullous lesions without pruritus |
116 (78.4%) 84 (72.4%) 32 (21.6%) 27 (84.3%) |
| Clinical manifestations at diagnosis bullous non-bullous urticaria-like features eczema-like features pruritus pain |
105 (71%) 43 (29%) 16 (37%) 27 (63%) 139 (94%) 31 (21%) |
| Distribution of skin lesions trunk limbs head and neck skin folds genitalia |
135 (91.2%) 111 (75%) 52 (35.1%) 14 (9.5%) 7 (4.7%) |
| Cutaneous involvement <10% 10-30% >30% |
43 (29%) 30 (20.3%) 75 (50.7%) |
| Mucosal involvement | 35 (23.6%) |
The median autoimmune bullous skin disorder intensity score at diagnosis was 52.5 (interquartile range,26-85.9). Of all patients, 145 (98%) had positive anti-BP180 antibodies and 70 (47.3%) had positive anti-BP230 antibodies, with median titres of 112.6 U/mL (interquartile range, 49.8-164.7 U/mL) and 48 U/mL (interquartile range, 2-99 U/mL) respectively.
Survival analysis
During a median follow-up period of 48 months (interquartile range, 11-150 months), 90 (60.8%) patients died, and 38 (42.2%) of them had active disease at the time of death. The cause of death was retrievable in all patients [Figure 1]. Cardiovascular diseases were the leading cause of death, seen in 40 (44.4%) patients. Three (3.3%) patients died of pemphigoid-related causes (sepsis due to skin infection with methicillin-resistant Staphylococcus aureus ) and they showed a lower overall survival as compared to the others (hazard ratio = 7.32 [95% confidence interval, 1.15-46.37], P = 0.035).
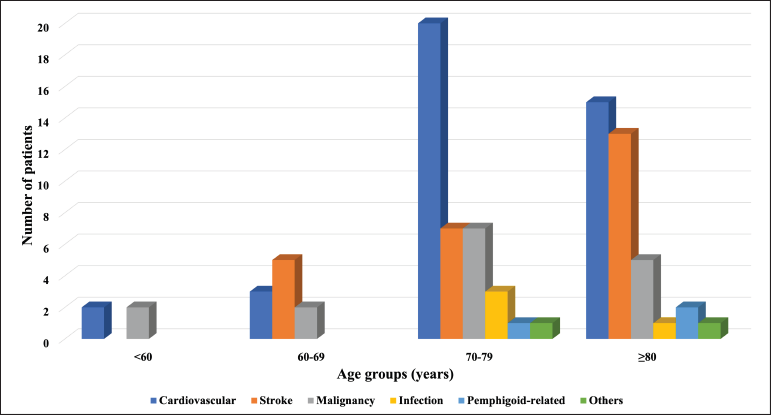
- Causes of death by age groups
The median overall survival was 17.5 months (interquartile range: 0-176). The Kaplan-Meier overall survival rates at 1, 3, 5 and 10 years were 74.2% (95% confidence interval, 67.5-81.6%), 53.4% (45.7-62.2%), 43.6% (35.9-53%) and 31.3% (23.5-41.7%). Only Kaplan-Meier curves for relevant prognostic factors (age ≥75 years, stroke, dementia, Alzheimer’s disease) have been illustrated [Figure 2]. We found no association between autoimmune bullous skin disorder intensity score and survival.
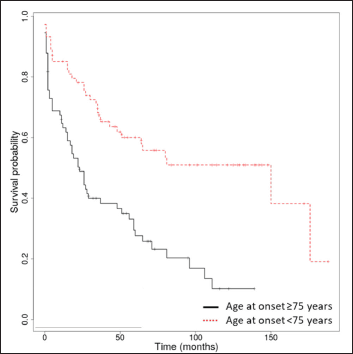
- Kaplan-Meier survival analysis in bullous pemphigoid patients (n = 148) with age over and under 75 years
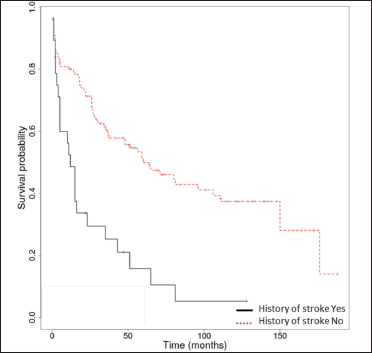
- Kaplan-Meier survival analysis in bullous pemphigoid patients (n = 148) with and without a history of stroke
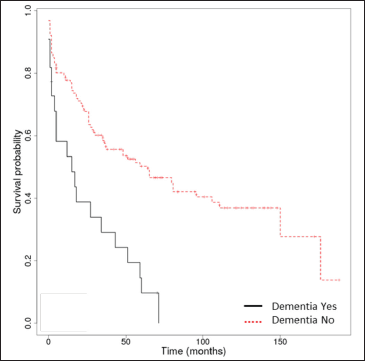
- Kaplan-Meier survival analysis in bullous pemphigoid patients (n = 148) with and without dementia
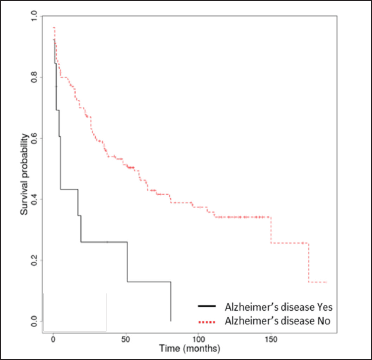
- Kaplan-Meier survival analysis in bullous pemphigoid patients (n = 148) with and without Alzheimer’s disease
Prognostic factors
Prognostic factors for overall survival among bullous pemphigoid patients which were statistically significant in the univariate and/or multivariate analysis (P < 0.05) are presented in Table 2. Age at onset ≥ 75 years was an independent risk factor for overall survival (hazard ratio = 1.07 [95% confidence interval, 1.05-1.1], P < 0.001).
| Characteristic | No. (%) (n = 148) | Deaths No. (%); (n = 90) Median (IQR) | Univariate HR (95% CI) | P-value uni variate Cox | Multivariate HR (95% CI) | P-value multi variate Cox |
|---|---|---|---|---|---|---|
| Age ≥75 years <75 years |
74 (50) 74 (50) |
56 (75.7) 34 (45.9) |
1.07 (1.05-1.1) |
<0.001 |
1.07 (1.05-1.1) |
<0.001* |
| Cutaneous involvement <10% 10-30% ≥30% |
43 (29.1) 30 (20.2) 75 (50.7) |
22 (51.1) 22 (73.3) 46 (61.3) |
2.16 (1.19-3.92) |
0.011 |
1.92 (1.01-3.67) |
0.047* |
| ABSIS | NA | NA | 52.5 (26-85.9) | 1 (1-1.01) | 1 (0.99-1.01) | 0.754 |
| ESR ≥30 mm/h <30 mm/h |
52 (35.1) 96 (64.9) |
37 (71.1) 53 (55.2) |
1.66 (1.08-2.53) |
0.02 |
− |
− |
| Skin infections Yes No |
17 (11.5) 131 (88.5) |
12 (70.6) 78 (59.5) |
1.7 (0.92-3.15) |
0.092 |
2.23 (1.15-4.34) |
0.018* |
| Cardiomyopathies Yes No |
8 (5.4) 140 (94.6) |
7 (87.5) 83 (59.3) |
2.79 (1.27-6.1) |
0.01 |
− |
− |
| Valvular heart disease Yes No |
18 (12.2) 130 (87.8) |
14 (77.8) 76 (58.5) |
2.16 (1.21-3.85) |
0.009 |
1.99 (1.07-3.69) |
0.03* |
| Chronic heart failure Yes No |
24 (16.2) 124 (83.8) |
19 (79.2) 71 (57.2) |
2.13 (1.28-3.57) |
0.004 |
− |
− |
| Neurological disease Yes No |
71 (48) 77 (52) |
61 (85.9) 29 (37.7) |
3.4 (2.21-5.24) |
<0.001 |
2.47 (1.57-3.88) |
<0.001* |
| History of stroke Yes No |
28 (19) 120 (81) |
24 (85.7) 66 (55) |
2.81 (1.74-4.55) |
<0.001 |
3.67 (2.2-6.11) |
<0.001* |
| Dementia Yes No |
22 (14.9) 126 (85.1) |
20 (90.9) 70 (55.5) |
2.77 (1.66-4.6) |
<0.001 |
− |
− |
| Alzheimer’s disease Yes No |
13 (8.8) 135 (91.2) |
11 (84.6) 79 (58.5) |
2.77 (1.46-5.26) |
0.002 |
− |
− |
| Malignancies Yes No |
19 (12.8) 129 (87.2) |
13 (68.4) 77 (59.7) |
1.51 (0.84-2.73) |
0.173 |
2.34 (1.25-4.4) |
0.008* |
| Topical corticosteroids Yes No |
29 (19.6) 66 (44.6) |
9 (31) 48 (72.7) |
0.68 (0.51-0.91) |
0.008 |
0.71 (0.53-0.96) |
0.025* |
| Statins Yes No |
32 (21.6) 116 (78.4) |
24 (75) 66 (56.9) |
1.63 (1.02-2.61) |
0.041 |
2.62 (1.58-4.34) |
<0.001* |
| Acetylsalicylic acid Yes No |
69 (46.6) 79 (53.4) |
51 (73.9) 39 (49.4) |
1.81 (1.19-2.75) |
0.006 |
− |
− |
Statistical significance at P < 0.05. ABSIS, Autoimmune Bullous Skin Disorder Intensity Score; CI, confidence interval; ESR, erythrocyte sedimentation rate; HR, hazard ratio; IQR, interquartile range
Cardiovascular diseases were the most common comorbidities in our cohort, being observed in 131 (88.5%) patients. In the univariate analysis, cardiomyopathies, valvular heart disease and chronic heart failure were found to be significantly associated with decreased overall survival. However, only valvular heart disease was detected as an independent risk factor for overall survival in the multivariate analysis (hazard ratio = 1.99 [95% confidence interval, 1.07-3.69], P = 0.03). No associations between survival and arterial hypertension, ischemic heart disease and arrhythmias were observed.
Among neurological diseases, stroke, non-Alzheimer’s dementia and Alzheimer’s disease were significantly associated with diminished overall survival in the univariate analysis. However, in the multivariate analysis, only neurological diseases assessed collectively (hazard ratio = 2.47 [95% confidence interval, 1.57-3.88], P < 0.001) and stroke (hazard ratio = 3.67 [95% confidence interval, 2.2-6.11], P < 0.001) were significant risk factors for overall survival in bullous pemphigoid patients, whereas Parkinson’s disease and epilepsy were not.
Malignancy (solid tumours such as breast cancer, colon cancer, Hodgkin lymphoma etc.) (hazard ratio = 2.34 [95% confidence interval, 1.25-4.4], P = 0.008) was found to be an additional risk factor for decreased overall survival in both univariate and multivariate analysis. Regarding other diseases present at the time of diagnosis viz., diabetes mellitus, and pulmonary, renal and thyroid diseases, no associations with overall survival were found.
Skin infections at diagnosis were associated with decreased overall survival in multivariate analysis (hazard ratio = 2.23 [95% confidence interval, 1.15-4.34], P = 0.018). Seventeen (11.5%) patients had bacterial skin infections with Staphylococcus spp., the latter being defined as the presence of purulent discharge over the wound and confirmed by skin culture performed at diagnosis. All these patients required systemic antibiotics. Three of them died of sepsis due to skin infection with methicillin-resistant Staphylococcus aureus within two months after diagnosis.
Median anti-BP180 and anti-BP230 autoantibodies were linked to lower overall survival, but only in the univariate analysis (hazard ratio = 1 [95% confidence interval, 1-1], P < 0.029), (hazard ratio = 1.01 [95% confidence interval, 1-1.01], P = 0.011) respectively, whereas patients with severe disease (≥30% body surface area) had a significantly lower overall survival with a multivariate risk of hazard ratio = 1.92 (95% confidence interval, 1.01-3.67), P = 0.047. The erythrocyte sedimentation rate was also found to be a prognostic factor (hazard ratio = 1.66 [95% confidence interval, 1.08-2.53], P = 0.02), but this did not persist in multivariate analysis.
Preceding medications
The most common drug classes used by bullous pemphigoid patients were those to treat cardiovascular diseases [Table 3]. Fourteen (9.5%) patients had no medicine intake at the time of diagnosis. In univariate analysis, statins and acetylsalicylic acid were associated with a lower overall survival. Nevertheless, in multivariate analysis, only statins remained an independent risk factor for lower survival (hazard ratio = 2.62 [95% confidence interval, 1.58-4.34], P < 0.001).
| Medications | Total number of patients (%) |
|---|---|
| Cardiovascular Beta blockers Calcium channel blockers ACE inhibitors Diuretics Acetylsalicylic acid Anticoagulants Statins |
81 (54.7) 28 (18.9) 67 (45.3) 58 (39.2) 69 (46.6) 4 (2.7) 32 (21.6) |
| Antidiabetics Metformin Gliptins |
18 (12.2) 9 (6.1) |
| Neuropsychiatric Cholinesterase inhibitors Benzodiazepines Antipsychotics |
14 (9.5) 5 (3.4) 5 (3.4) |
| NSAIDs | 12 (8.1) |
| No medications | 14 (9.5) |
ACE inhibitors, angiotensin-converting enzyme inhibitors; NSAIDs, non-steroidal anti-inflammatory drugs
Bullous pemphigoid treatment
With regard to bullous pemphigoid-specific therapy, 78 (52.7%) patients received dual corticosteroid therapy (oral-prednisone, and topical-clobetasol), 66 (44.6%) received oral corticosteroids alone and only four (2.7%) received topical corticosteroids exclusively. The median initial dose of oral corticosteroids was 0.49 mg/kg/day (interquartile range, 0.37-0.62). Thirty-two (21.6%) patients received besides oral corticosteroids, azathioprine (n = 21), dapsone (n = 7), methotrexate (n = 1) and azathioprine + dapsone (n = 3). Antihistamines were used in 74 (50%) patients.
Bullous pemphigoid patients’ survival was further evaluated based on the therapeutic regimen. The use of topical corticosteroids was independently and positively associated with increased survival (hazard ratio = 0.71 [95% confidence interval, 0.53-0.96], P = 0.025). Oral corticosteroids, other immunosuppressants and antihistamines showed no influence on the survival of these patients.
Discussion
We retrospectively evaluated 148 bullous pemphigoid patients for a median follow-up period of 48 months. This is one of the longest follow-up studies conducted on bullous pemphigoid patients in Europe. So far, only the study of Cortés et al. reported a 5-year survival rate.12 In line with previously published data, the leading causes of death were cardiovascular and cerebrovascular diseases.5,6,13 Of note, three (3.3%) patients died of pemphigoid-related causes, showing significantly lower overall survival rates as opposed to those who died of other causes. They developed extensive, refractory forms of bullous pemphigoid and died of sepsis with methicillin-resistant Staphylococcus aureus.
We also sought to identify potential prognostic factors for overall survival in bullous pemphigoid. The median age of 74.5 years in our cohort was comparable with that in previous studies.8,14,15 Likewise, we identified older age (≥75 years) as a risk factor for overall survival in the multivariate analysis.5,6,8,14,15
In our study, extensive cutaneous involvement (≥30% body surface area) was significantly associated with lower overall survival. This finding was reported only in two other studies.8,16 Notwithstanding, a meta-analysis regarding prognostic factors for mortality in bullous pemphigoid patients did not show any significant association.17 A possible explanation for this discrepancy is the subjectivity and intra- and interobserver variability associated with body surface area-based severity assessment. On the other hand, the autoimmune bullous skin disorder intensity score is also not specific for any particular autoimmune blistering dermatosis.11 Correspondingly, we found no association between autoimmune bullous skin disorder intensity score and survival.3,6,15,17 Recently, the novel bullous pemphigoid disease area index score was proposed and validated, which is both specific and objective for disease severity assessment in bullous pemphigoid.18
Particularly noteworthy was the association between skin infections in denuded bullae and lower overall survival. It has been shown that infections are common in bullous pemphigoid, especially among patients with functional impairment and dementia, thus increasing mortality.19,20 The most common infections in bullous pemphigoid patients are found in the skin and mucosa.21 In our cohort, all detected skin infections were staphylococcal and required systemic antibiotics. Of these, three (3.3%) patients died of sepsis with methicillin-resistant Staphylococcus aureus. As far as we know, this is one of the few studies to look at the prognostic role of bacterial skin infections at diagnosis in the survival of bullous pemphigoid patients.
Cardiac and neurological comorbidities were also linked to decreased overall survival. Cardiac diseases such as ischemic heart disease and heart failure were reported as risk factors for death in bullous pemphigoid subjects.15,20,22,23 We identified cardiomyopathies, valvular heart disease and chronic heart failure as poor prognostic factors for bullous pemphigoid patients in the univariate analysis. However, in the multivariate analysis, the association persisted only for valvular heart disease. We were unable to find any previous reports regarding the role of valvular heart disease in the prognosis of bullous pemphigoid. Interestingly, valvular heart disease as a result of chronic inflammation and scarring of the endothelium was also prevalent in other autoimmune diseases.24 It is therefore tempting to speculate that the many cellular players involved in the inflammatory processes of bullous pemphigoid, might also be involved in the valvular damage, thus increasing the mortality of these patients.25 However, further studies are needed to evaluate this association and corroborate if patients with bullous pemphigoid have indeed a higher risk of developing valvular heart disease as compared to the general population.
Consistent with previously published data, we found a strong association between neurological disorders and decreased survival. Neurological diseases assessed collectively, and a history of stroke were identified as poor prognostic factors. Similar to other studies, dementia and Alzheimer’s disease were associated with decreased overall survival only in the univariate analysis.20,26 The association between bullous pemphigoid and neurological disorders is not yet fully understood. A proposed hypothesis is that BP180 and/or BP230 autoantibodies cross-reactively target the namesake neuronal isoforms, which are expressed in the central nervous system.1 Interestingly, a previous study showed that patients with bullous pemphigoid have a 3.6-fold increased risk for neurological disorders prior to the diagnosis of bullous pemphigoid, raising the issue of the exposure of neuronal isoforms as triggers for bullous pemphigoid development.27
In our study, malignancy was found as a significant prognostic factor in the prediction of survival for bullous pemphigoid. The relationship between bullous pemphigoid and cancer still remains controversial. In a 10-year population-based study, no increased risk of cancer in bullous pemphigoid patients has been shown.28 Conversely, there is evidence that suggests an increase in hematologic malignancies among bullous pemphigoid patients.29 A possible explanation for an association with cancer is the cross-reactivity of antibodies directed against tumour-specific antigens of malignant cells with basement membrane bullous pemphigoid antigens.28 However, no cancer screening is currently recommended for bullous pemphigoid patients at diagnosis.
Comparable to other published reports, we did not find diabetes, lung, kidney and thyroid disease as risk factors for lethal outcomes among bullous pemphigoid patients.6,8,15 Furthermore, we found that elevated erythrocyte sedimentation rate was significantly associated with lower overall survival, but this did not persist in the multivariate analysis, analogous to other studies.5,8,16,20
Remarkably, we found a positive correlation between the use of topical corticosteroids, either alone or combined with oral corticosteroids and overall survival among bullous pemphigoid patients. However, we did not find oral corticosteroids or other immunosuppressive agents, as predictive factors for survival in bullous pemphigoid. Conversely, a large prospective trial by Joly et al. showed a greater mortality rate and higher corticosteroid-associated side effects among patients treated with oral prednisone, compared with those treated with topical corticosteroids.30 This study changed the traditional paradigm of therapy, with topical corticosteroids being currently recommended as the first line of treatment for bullous pemphigoid.31 Nonetheless, the protective effects of topical corticosteroids as monotherapy could also be due to their use in milder forms of bullous pemphigoid. In our study, almost every patient received either dual or oral corticosteroid treatment, with only four (2.7%) subjects receiving topical treatment alone.
Contrary to the study of Rozenblat et al., we found that the use of statins was associated with lower overall survival rates among bullous pemphigoid patients.26 Interestingly, a more recent well-matched population-based cohort study with appropriate follow-up time did not show any significant association between the use of statins and the risk of developing bullous pemphigoid.32 It is noteworthy that in our study 131 (88.5%) patients had cardiovascular diseases, which were also the leading cause of death, followed by stroke. Therefore, many of the patients were taking statins at the time of diagnosis. A similar explanation might be feasible for acetylsalicylic acid, which was also associated with lower survival. Moreover, its daily use in healthy older adults was linked to higher all-cause mortality, mainly attributed to cancer-related deaths, but also to gastrointestinal side effects.33 However, in our cohort this correlation did not persist after adjustment for confounders.
The strengths of this study are: (1) complete diagnostics were performed in all patients, and only those with positive direct immunofluorescence microscopy and positive enzyme-linked immunosorbent assay BP180 and/or BP230 immunoglobulin G autoantibodies were included; (2) the long observation period of up to 18 years in the same geographical area; (3) extensive data regarding demographic, bullous pemphigoid characteristics, comorbidity profiles and types of treatments were recorded; (4) follow-up data were included for every patient; missing data about the date and cause of death was obtained from national registers and (5) we adjusted the analyses for important known confounders.
This study lacks a control cohort to validate the obtained results, since the majority of bullous pemphigoid patients are elderly and already have many associated comorbidities. Other study limitations include the retrospective document-based design and that it was based in a single institution, though this was a referral centre for autoimmune bullous diseases. In addition, indirect immunofluorescence microscopy was not performed in all patients.
Conclusion
This is the first study assessing survival and prognostic factors of bullous pemphigoid in Romania. Advanced age, extent of skin involvement, cardiac and neurological diseases and malignancy were identified as predictors of decreased survival in bullous pemphigoid. We found for the first time that valvular heart disease and skin infections at diagnosis were independent risk factors for decreased survival in bullous pemphigoid. We also observed that the use of topical corticosteroids was associated with increased overall survival, whereas the use of statins was linked to lower survival rates. Our findings may aid dermatologists in the reduction of bullous pemphigoid mortality by identifying high-risk patients and managing them accordingly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This article was supported by the internal doctoral grant number 2461/53/17.01.2020 of the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Conflict of interest
There are no conflicts of interest.
References
- The growing incidence of bullous pemphigoid: Overview and potential explanations. Front Med (Lausanne). 2018;5:220.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prediction of survival for patients with bullous pemphigoid. Arch Dermatol. 2005;141:691-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pemphigoid and pemphigus vulgaris-incidence and mortality in the UK: Population based cohort study. BMJ. 2008;337:a180.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality of bullous pemphigoid in Switzerland: A prospective study. Br J Dermatol. 2011;165:368-74.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality of bullous pemphigoid in the first year after diagnosis: A retrospective study in a Spanish medical centre. J EurAcad Dermatol Venereol. 2014;28:500-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998-2004.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality in bullous pemphigoid and prognostic factors in 1st and 3rd year of follow-up in specialized centre in Poland. Arch Dermatol Res. 2017;309:709-19.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for lethal outcome in patients with bullous pemphigoid. Arch Dermatol. 2002;138:903-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective study in bullous pemphigoid: Association of high serum anti-BP180 IgG levels with increased mortality and reduced Karnofsky score. Br J Dermatol. 2018;179:918-24.
- [CrossRef] [PubMed] [Google Scholar]
- Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17:4-11.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality rate in bullous pemphigoid: A retrospective monocentric cohort study. Dermatology. 2012;225:320-25.
- [CrossRef] [PubMed] [Google Scholar]
- Anti BP180-autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J EurAcad Dermatol Venereol. 2020;34:1583-9.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014;71:92-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mortality of bullous pemphigoid: An evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol. 2008;59:582-8.
- [CrossRef] [PubMed] [Google Scholar]
- High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol. 1998;134:465.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors for mortality in patients with bullous pemphigoid: A meta-analysis. Arch Dermatol Res. 2017;309:335-47.
- [CrossRef] [PubMed] [Google Scholar]
- International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-12.
- [CrossRef] [PubMed] [Google Scholar]
- Infectious complications in bullous pemphigoid: An analysis of risk factors. J Am Acad Dermatol. 2015;72:834-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality of bullous pemphigoid in Singapore: Risk factors and causes of death in 359 patients seen at the National Skin Centre. Br J Dermatol. 2014;170:1319-26.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the Characteristics and associated factors of infectious complications in bullous pemphigoid. Front Immunol. 2020;11:1607.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment and mortality rate of bullous pemphigoid in China: A hospital-based study. Eur J Dermatol. 2013;23:94-8.
- [CrossRef] [PubMed] [Google Scholar]
- Survival prognosis in pemphigoid. A cohort analysis of 78 patients. Ann Dermatol Venereol. 1995;122:751-7.
- [PubMed] [Google Scholar]
- Autoimmune valvular carditis. Curr Allergy Asthma Rep. 2015;15:491.
- [CrossRef] [PubMed] [Google Scholar]
- Coagulation and skin autoimmunity. Front Immunol. 2019;10:1407.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality and risk factors among Israeli bullous pemphigoid patients. Arch Dermatol Res. 2019;311:19-27.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br J Dermatol. 2011;165:593-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer is not a risk factor for bullous pemphigoid: 10-year population-based cohort study. Br J Dermatol. 2019;180:553-8.
- [CrossRef] [PubMed] [Google Scholar]
- Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol. 2015;135:1445-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321-7.
- [CrossRef] [PubMed] [Google Scholar]
- Management of bullous pemphigoid: The European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867-77.
- [CrossRef] [PubMed] [Google Scholar]
- The association between statins and subsequent risk of bullous pemphigoid: A population-based cohort study. JAAD Int. 2021;3:23-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
- [CrossRef] [PubMed] [Google Scholar]






