Translate this page into:
Symmetrical drug-related intertriginous and flexural exanthema-like eruption: An addition to the spectrum of coronavirus disease 2019-related cutaneous findings
Corresponding author: Dr. Özlem Akın Çakıcı, Marmara Universitesi Pendik Egitim ve Arastirma Hastanesi Dermatoloji Anabilim Dali, Pendik, Istanbul, Turkey. ozlm_akin@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Çakıcı ÖA, Güder S, Salman A, Ergun T. Symmetrical drug-related intertriginous and flexural exanthema-like eruption: An addition to the spectrum of coronavirus disease 2019-related cutaneous findings. Indian J Dermatol Venereol Leprol 2022;88:814-6.
Sir,
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is a distinctive form of cutaneous adverse drug reaction which occurs following systemic exposure to a drug via transcutaneous, oral, parenteral or inhalational routes.1 Till now, only a few cases of SDRIFE-like rashes have been reported in the setting of coronavirus disease 2019 (COVID-19) or vaccination.2-5 We aim to discuss its relation to COVID-19 and vaccination with this series of three patients.
The first case was a 25-year-old man, otherwise healthy healthcare worker, presenting with a severe pruritic rash that developed one day after the administration of the first dose of the Pfizer-BioNTech vaccine. He had no systemic symptoms, including those suggestive of COVID-19. His medical history was insignificant and did not reveal any recent exposure to drugs or herbal medicines. Dermatological examination disclosed symmetrical erythema involving both groins [Figures 1a], palmoplantar areas [Figure 1b] and axillary folds [Figure 1c]. Laboratory tests including complete blood count, erythrocyte sedimentation rate, liver transaminases, serum creatinine and urea were within normal range. Serological tests ruled out rubella, rubeola and syphilis and infections due to parvovirus B19, Epstein-Barr virus, cytomegalovirus and hepatitis B virus. The nasal swab reverse transcription polymerase chain reaction test for COVID-19 could not be performed because as per recommendation of National Ministry of Health, its use was restricted to patients with symptoms suggestive of COVID-19.6 The patient refused skin biopsy. The patient fulfilled the diagnostic criteria for symmetrical drug-related intertriginous and flexural exanthema.1 Treatment with mometasone furoate 0.1% cream and 0.5 mg/kg methylprednisolone per oral tapered within ten days led to resolution of the lesions. The patient had the second dose of the same vaccine six months later without any recurrences. The patient had a score of six on Naranjo adverse drug reaction probability scale, indicating probable adverse drug reaction.7
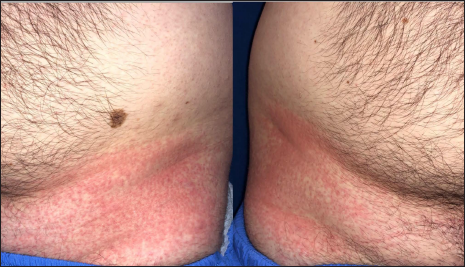
- Patient 1: Bilaterally symmetrical erythema on the groins
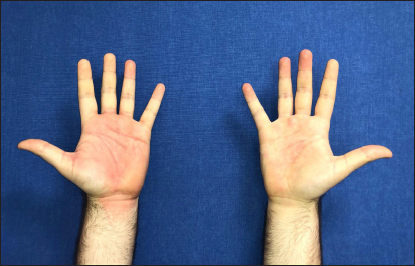
- Patient 1: Erythema involving both palms
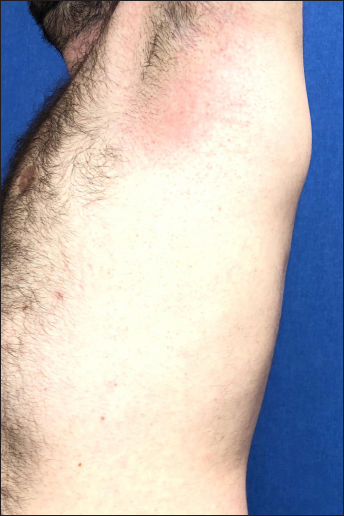
- Patient 1: Erythema on the axilla
The second case was a 51-year-old lady who presented with an itchy rash that developed two days after her second dose of the Pfizer-BioNTech vaccine. A history of drug or herbal product exposure or symptoms suggestive of coronavirus disease 2019 was not present. Physical examination showed erythematous papules and plaques affecting axillary, inframammary areas and gluteal folds in a symmetric distribution consistent with SDRIFE. According to Naranjo adverse drug reaction probability scale, the patient had a score of seven, indicating a probable drug reaction.7 The lesions resolved following the treatment with 32 mg/day methylprednisolone per oral tapered and stopped over two weeks.
The third case, a 60-year-old lady, had a positive nasal swab reverse transcription polymerase chain reaction test for COVID-19. Chest computed tomography revealed bilateral ground-glass opacities, consistent with a diagnosis of COVID-19 pneumonia.5 Her personal medical history was insignificant. She was treated by Favipiravir as per the national guidelines.5 On the third day of the treatment erythema involving the inguinal region [Figure 2], axillary folds [Figure 3], gluteal areas and popliteal fossae were noticed. Favipiravir was discontinued and treatment with clobetasol propionate 0.05% ointment twice a day led to resolution of the lesions within two weeks. Patient had a score of four on Naranjo adverse drug reaction probability scale, suggestive of possible drug reaction.7 However, it should also be noted that concomitant coronavirus infection may also be responsible for the rash.
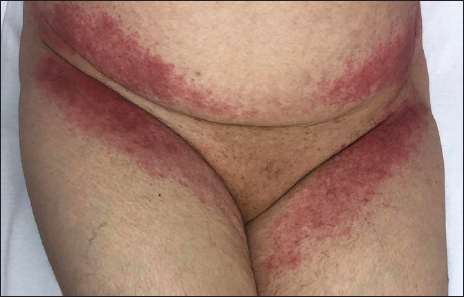
- Patient 3: Bilaterally symmetrical erythema on the inguinal region
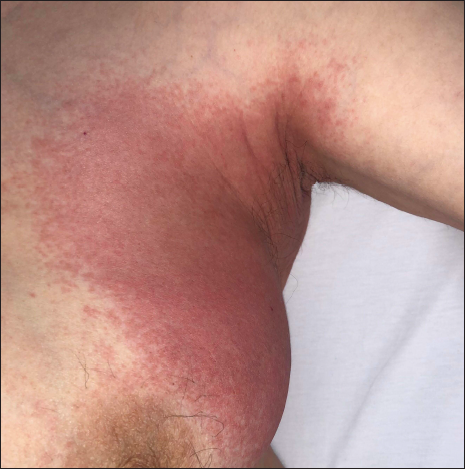
- Patient 3: Erythema on the axilla
Symmetrical drug-related intertriginous and flexural exanthema was first described by Hausserman et al. in 2004 as a delayed-type adverse drug reaction caused by systemic medications. The diagnostic criteria proposed are: (1) exposure to the systemic drug at first or repeated dose (2) erythema of the gluteal/perianal area and/or v-shaped erythema of the inguinal/perigenital area (3) involvement of at least one other intertriginous/flexural localization (4) symmetrical involvement in relevant areas and (5) absence of systemic symptoms.1 It usually occurs within two days of exposure to the offending agent.1 The most common relevant drugs can be listed as beta-lactam antibiotics, aminopenicillins, radiocontrast media, chemotherapeutics, biological agents and antihypertensives.1 Diagnosis is based on history and clinical symptoms, as histopathological features are non-specific.1 The skin patch testing is negative in half of the cases, though re-challenge by the initiating agent is positive in most of the cases and confirms the diagnosis.1
Vaccines can also trigger symmetrical drug-related intertriginous and flexural exanthema. Only a few cases occurring after the COVID-19 vaccination have been reported so far. Two case reports of SDRIFE occurring after recombinant adenoviral vector vaccine (AstraZeneca-Oxford), which contains the excipient polysorbate 80, have been published.2,3 Also, Orenay et al. reported another patient with a SDRIFE-like eruption developed following the administration of inactivated coronavirus vaccine containing aluminum hydroxide (CoronaVAC).4 We found only three previous reports of SDRIFE related to mRNA vaccines (Pfizer-BioNTech).8,9 The mRNA vaccines were developed with brand-new technology and contain the excipient polyethylene glycol, which may be responsible for early and late hypersensitivity reactions associated with mRNA vaccines.8 Our first patient developed SDRIFE following the first dose of Pfizer-BioNTech vaccine, but tolerated the second dose without any adverse events. A similar observation was made by McMahon, et al. They found that only 43% of patients who developed an adverse reaction following the first dose of Moderna or Pfizer COVID-19 vaccination manifested a recurrence after the second dose.10 Taken together, three different vaccines are claimed to trigger SDRIFE, highlighting the importance of bearing this rare reaction in mind within the repertory of COVID-19 vaccine-related cutaneous reactions.
Other than vaccination, a symmetrical drug-related intertriginous and flexural exanthema-like rash was also reported in two patients with COVID-19. While one of them was on treatment with piperacillin/tazobactam, azithromycin, hydroxychloroquine and oseltamivir while the other developed SDRIFE following remdesivir.5,11 However, it is not clear whether the rash is associated with COVID-19 or the drugs in these cases. In our third patient, favipiravir is more likely the culprit because the rash developed on the third day of treatment. Active viral infection may have contributed to the emergence of drug eruption.5
Despite the intensive vaccination programs against COVID-19, the pandemic is ongoing with new cases and variants. New vaccines and drugs may be expected to be added to the therapeutic armamentarium as well as prophylaxis. Meanwhile, careful documentation and further research will allow a better understanding of the pathophysiology of this rare reaction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): A closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-80.
- [CrossRef] [PubMed] [Google Scholar]
- Symmetrical drug-related intertriginous and flexural exanthema like eruption associated with COVID-19 vaccination. Clin Exp Dermatol. 2022;47:175-6.
- [CrossRef] [PubMed] [Google Scholar]
- Symmetric drug-related intertriginous and flexural exanthema-like eruption related to coronavirus disease 2019 vaccine. Contact Dermatitis. 2022;87:91-3.
- [CrossRef] [Google Scholar]
- Systemic drug-related intertriginous and flexural exanthema like eruption after CoronaVac vaccine. J Eur Acad Dermatol Venereol. 2021;35:e634-35.
- [CrossRef] [PubMed] [Google Scholar]
- SDRIFE-like rash in COVID-19 patient: Drug reaction or another cutaneous manifestation of SARS-CoV-2? Int J Dermatol. 2021;60:884-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ankara: TR Ministry of Health; [cited 2020 Oct 12] Available from: https://covid19.saglik.gov.tr/TR-66301/covid-19-rehberi.html
- A method for estimating the probability of adverse drug reactions. Clin Pharacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic drug-related intertriginous and flexural exanthema induced by the Pfizer-BioNTech COVID-19 vaccine: A report of 2 cases. JAAD Case Rep. 2021;18:57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- [CrossRef] [PubMed] [Google Scholar]
- Remdesivir-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE)? A case report with review of the literature. Eur J Clin Pharmacol. 2021;77:141-4.
- [CrossRef] [PubMed] [Google Scholar]





