Translate this page into:
Systemic sarcoidosis mimicking metastases in a patient with breast cancer: A misdiagnosis resolved by the appearance of skin lesions
2 Department of Internal Medicine, Seoul National University Hospital; Cancer Research Institute, Seoul National University, Seoul, Korea
Correspondence Address:
Je-Ho Mun
Department of Dermatology, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080
Korea
| How to cite this article: Lee H, Cho SI, Lee KH, Mun JH. Systemic sarcoidosis mimicking metastases in a patient with breast cancer: A misdiagnosis resolved by the appearance of skin lesions. Indian J Dermatol Venereol Leprol 2020;86:711-715 |
Sir,
Sarcoidosis can mimic other disease processes and it can be easily misdiagnosed as another inflammatory disease or a malignancy.[1] Here we present a case of breast cancer whose accurate cancer staging was confounded by the concomitant presence of previously undiagnosed sarcoidosis.
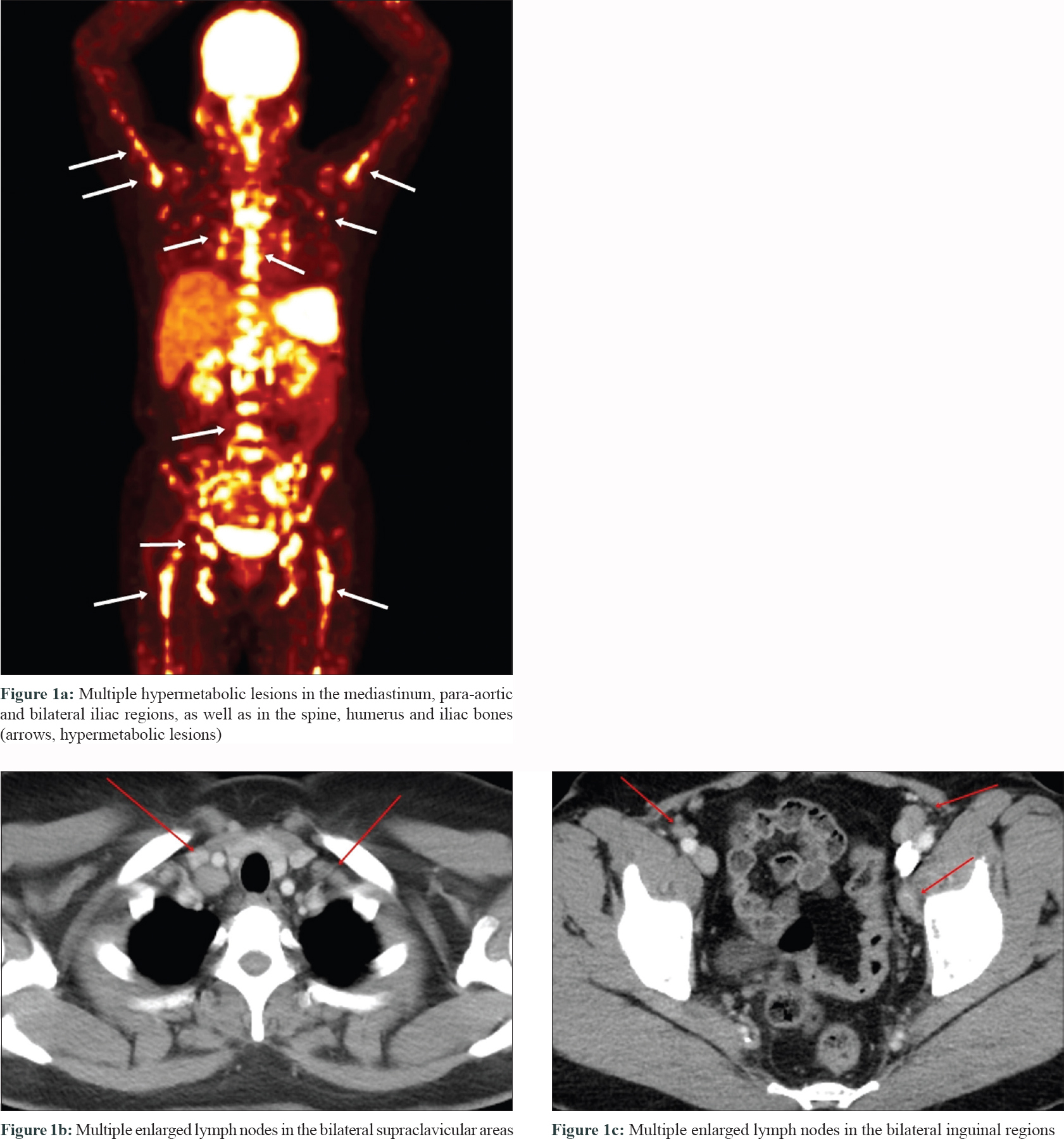
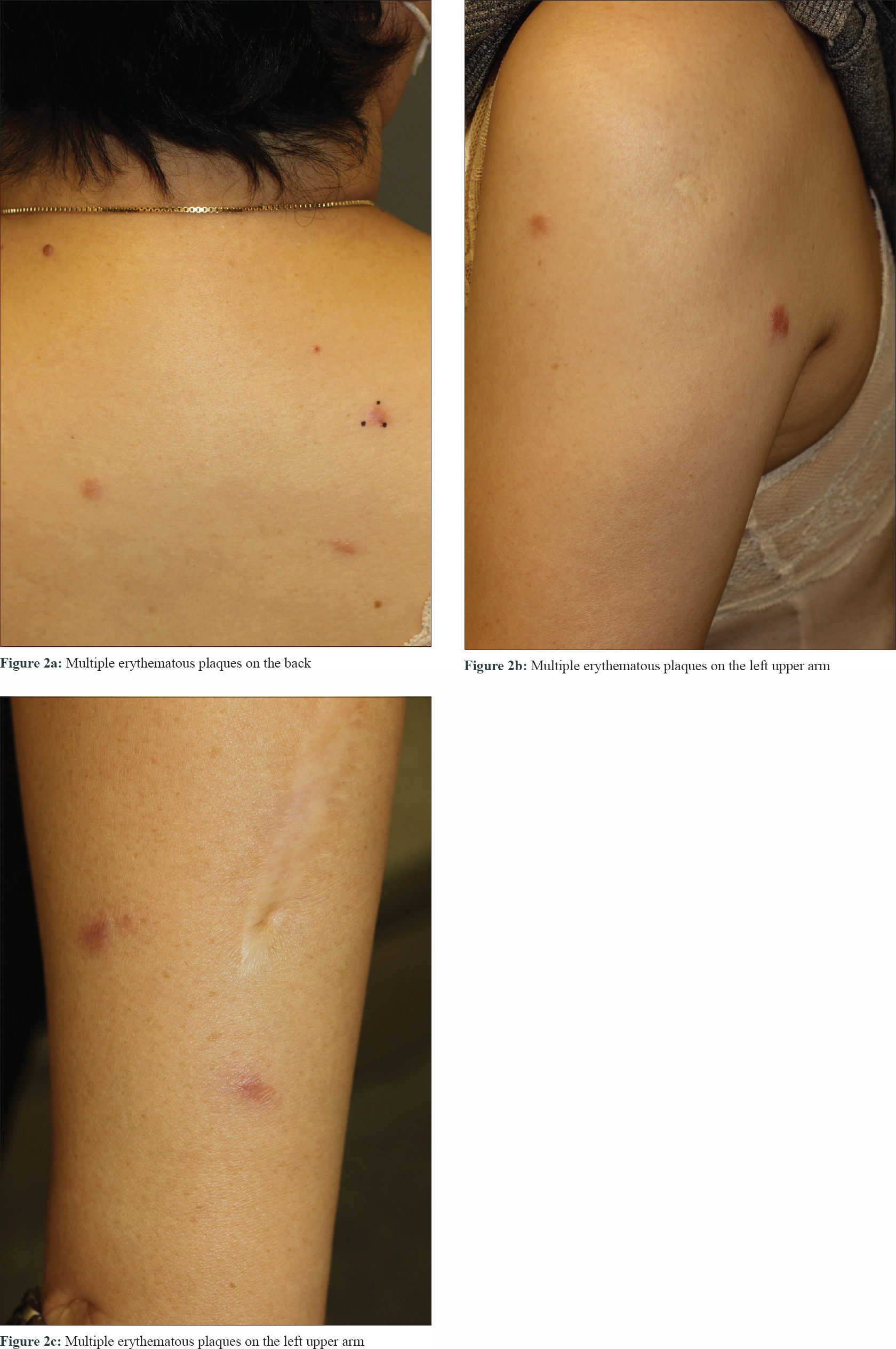
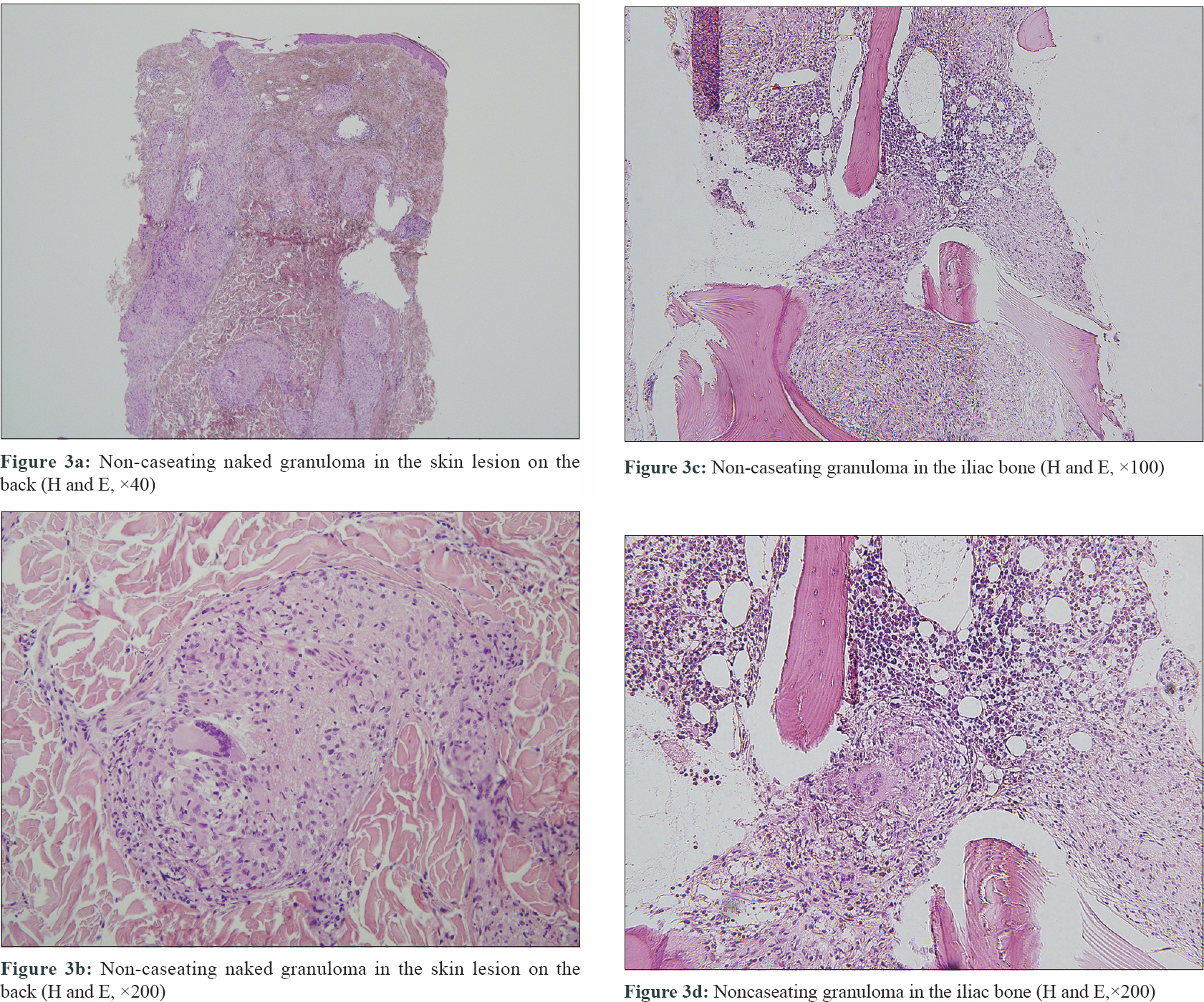
A 51-year-old woman who had received multiple chemotherapy regimens for metastatic breast cancer presented to the dermatology clinic with an erythematous skin eruption. She was initially diagnosed with triple-negative invasive ductal carcinoma of the right breast via core needle biopsy 1 year back. An ultrasound examination showed a right breast mass (3.9 × 1.7 × 2.6 cm) and multiple enlarged left axillary and bilateral supraclavicular lymph nodes. Besides, [(18) F]-Fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography confirmed additional enlarged lymph nodes in the mediastinum, retroperitoneum and bilateral inguinal regions, as well as hypermetabolic nodules in the spine and iliac bones [Figure - 1]a, [Figure - 1]b, [Figure - 1]c. With a presumed diagnosis of metastatic breast cancer, the patient underwent 16 sessions of chemotherapy for a period of 10 months at 2–3-week intervals. The breast mass showed a partial reduction in size (2.4 × 1.5 × 2.1 cm) on computed tomography of the chest and ultrasound examination at 3 months of chemotherapy. However, the other presumed metastatic lesions remained unchanged on multiple computed tomography and [(18) F]-Fluoro-2-deoxy-D-glucose-positron emission tomography during the 10-month treatment course. Moreover, there were multiple nodules found in the lungs during the treatment course. Subsequently there were appearance of multiple erythematous plaques on the back [Figure - 2]a, left arm [Figure - 2]b and [Figure - 2]c and left thigh. Skin biopsy from a plaque on the back showed naked granulomas indicating sarcoidosis [Figure - 3]a and [Figure - 3]b. Further biopsies of the left axillary lymph node and left iliac [Figure - 3]c and [Figure - 3]d and L5 spinal bones also showed features of sarcoidosis without any evidence of malignancy. Her serum angiotensin-converting enzyme was elevated at 150 U/L (normal range, 7.5–53.0 U/L) and pulmonary function tests revealed reduced diffusing capacity of the lung for carbon monoxide. Based on the above findings the patient was diagnosed with multisystem sarcoidosis. Another core needle biopsy conducted on the aforementioned remnant right breast mass also showed only noncaseating granuloma without any cancer cells. Nevertheless, since a core needle biopsy represents only a small part of the lesion, it was unclear whether the malignant carcinoma responded completely to chemotherapy. After a thorough consultation, the patient denied further chemotherapy and opted for regular radiologic work-ups at 2–3 months intervals.
 |
 |
 |
She was started on oral prednisolone for sarcoidosis at an initial dose of 15 mg/day with a resolution of skin lesions within the first couple of months of treatment. However, other systemic sarcoid lesions remained unchanged necessitating an increase in the dose to 40 mg/day at 3 months which was gradually tapered. A follow-up computed tomography scan at 9 months (prednisolone dose was 5mg/day at this time) into the oral steroid treatment showed a reduction in the dimensions of the lymph nodes and lung nodules. The bone scan showed no evidence of bone lesions. However, at this time, the computed tomography scan and an ultrasound examination revealed an increased primary breast lesion size (3.4 × 1.6 × 2.3 cm) and its biopsy was confirmed as invasive ductal carcinoma. The patient underwent total mastectomy with axillary lymph node dissection. A histopathologic examination revealed the presence of invasive ductal carcinoma with clear surgical margins. The patient received postoperative adjuvant chemotherapy consisting of 8 cycles of capecitabine (1500 mg). Systemic prednisolone was stopped after another 3 months of treatment. She is now on a regular follow-up schedule at 3 months intervals to monitor both sarcoidosis and breast cancer. The most recent computed tomography scan done 10 months postoperatively showed no signs of metastasis and sarcoidosis and the cutaneous lesions have not recurred so far.
Sarcoidosis is a benign systemic granulomatous disease of unknown etiology that affects multiple organs.[2] When coexisting with a primary diagnosis of cancer, the presence of sarcoidosis may complicate the accurate staging of cancer. The concomitant presence of cancer and sarcoidosis may induce high uptakes of [(18) F]-Fluoro-2-deoxy-D-glucosein the lymph nodes or bones.[3] Besides, the lung and mediastinal lymph nodes are frequently enlarged in patients with sarcoidosis as well as in those with metastatic breast cancer.[3] In our case, the patient had enlarged lymph nodes in the left axilla and bilateral supraclavicular areas which are other common sites involved in metastatic breast cancer.[3],[4]
Although the initial clinical and radiological findings in the present case were highly suggestive of metastatic breast cancer, if the axillary lymph node biopsy had been performed initially, the delay in the correct diagnosis might have been averted. There have been several case reports demonstrating the coexistence of sarcoidosis in patients with breast cancer.[1],[3],[4],[5] However, the majority of these reports described a typical presentation of sarcoidosis as lesions were limited to the lungs and mediastinal lymph nodes without bone involvement. The patient described in this case report had enlarged mediastinal lymph nodes as well but this was an exceptional case that also involved the bones, axillary and supraclavicular lymph nodes. Without the skin manifestation and its histological analysis, the accurate diagnosis and treatment could have been further delayed.
In conclusion, this case highlights the diagnostic dilemma due to the concomitant presence of sarcoidosis and breast cancer. Histological confirmation should always be considered before treatment is initiated, as it can change the staging and treatment plan.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Spiekermann C, Kuhlencord M, Huss S, Rudack C, Weiss D. Coexistence of sarcoidosis and metastatic lesions: A diagnostic and therapeutic dilemma. Oncol Lett 2017;14:7643-52.
[Google Scholar]
|
| 2. |
Boffetta P, Rabkin CS, Gridley G. A cohort study of cancer among sarcoidosis patients. Int J Cancer 2009;124:2697-700.
[Google Scholar]
|
| 3. |
Akhtari M, Quesada JR, Schwartz MR, Chiang SB, Teh BS. Sarcoidosis presenting as metastatic lymphadenopathy in breast cancer. Clin Breast Cancer 2014;14:e107-10.
[Google Scholar]
|
| 4. |
Chen J, Carter R 3rd, Maoz D, Tobar A, Sharon E, Greif F. Breast cancer and sarcoidosis: Case series and review of the literature. Breast Care (Basel) 2015;10:137-40.
[Google Scholar]
|
| 5. |
Altınkaya M, Altınkaya N, Hazar B. Sarcoidosis mimicking metastatic breast cancer in a patient with early-stage breast cancer. Ulus Cerrahi Derg 2016;32:71-4.
[Google Scholar]
|
Fulltext Views
7,872
PDF downloads
1,494





