Translate this page into:
Temozolomide-induced drug rash with eosinophilia and systemic symptoms syndrome
Corresponding author: Dr. Keshavamurthy Vinay, Associate Professor, Department of Dermatology, Venereology & Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mehta H, Gendle CS, Kumaran MS, Vinay K. Temozolomide-induced drug rash with eosinophilia and systemic symptoms syndrome. Indian J Dermatol Venereol Leprol 2023;89:160.
Sir,
Drug rash with eosinophilia and systemic symptoms syndrome (DRESS syndrome) is a rare and potentially life-threatening hypersensitivity reaction that presents with cutaneous rash, lymphadenopathy, eosinophilia and internal organ involvement.1 Temozolomide is an alkylating agent most often used for the management of high-grade gliomas. Several cutaneous adverse effects of temozolomide have been reported.2 However, temozolomide is not well-known as a causative agent of DRESS syndrome. Herein we report a case of DRESS syndrome in a patient of glioblastoma multiforme receiving temozolomide.
A 59-year-old man sought dermatology consultation with complaints of facial swelling and rash of five days duration. He was an operated case of glioblastoma multiforme of left superior frontal region receiving a combination of temozolomide (75 mg/m2 orally) and radiotherapy (6000 Gy) for the last 16 days. He was also on oral phenytoin 100 mg thrice a day from two months.
On examination, he was febrile (39ºC). There was facial oedema, widespread blanchable maculopapular rash [Figure 1], and non-tender axillary and inguinal lymphadenopathy. Investigations revealed leucocytosis (12,600 cells/mm3) with an absolute eosinophil count of 2,431 cells/mm and atypical lymphocytes in peripheral smear. His total and conjugated bilirubin levels were 2.5 mg/dL (normal: 0.3–1.2 mg/dL) and 0.7 mg/dL (normal: <0.3 mg/dL), respectively. Liver transaminases, renal parameters, muscle and pancreatic enzymes were normal. Our case fulfilled the registry of severe cutaneous adverse reactions criteria for diagnosis of DRESS syndrome and validation score was 7 [Table 1], suggesting “definite” DRESS syndrome. Algorithm for assessment of drug causality in epidermal necrolysis score was calculated to be 5 (“probable”) and 1 (“unlikely”) for phenytoin and temozolomide respectively [Table 2].
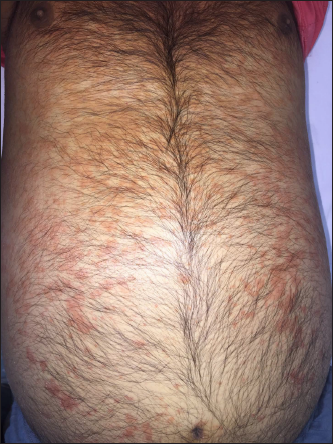
- Maculopapular rash involving the trunk
| Clinical manifestations | Score | Present case | ||||
|---|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | Value | Score | |
| General | ||||||
| Fever ≥ 38.5ºC | No/unknown | Yes | − | − | 39ºC | 0 |
| Enlarged lymph nodes | − | No/unknown | Yes | Inguinal and axillary lymphadenopathy | 1 | |
| Eosinophilia | ||||||
| Eosinophil count | − | No eosinophilia | 0.7–1.49 x 109/L |
>1.5 x 109/L |
Absolute eosinophil count = 2431 cells/mm3 | 2 |
| Eosinophil (%), if leucocytes count <4.0x109/L | − | − | 10–19.9% | ≥20% | ||
| Atypical lymphocytes | − | No/unknown | Yes | − | Yes | 1 |
| Skin involvement | ||||||
| Extent (% of body surface area) | − | No/unknown | >50% | − | 60% body surface area involved | 1 |
| Skin rash suggesting drug rash with eosinophilia and systemic symptoms | No | Unknown | Yes | − | Yes (facial oedema and infiltration) | 1 |
| Skin biopsy suggesting drug rash with eosinophilia and systemic symptoms | No | Yes/unknown | Not done | 0 | ||
| Organ involvement (score 1: 1 organ involvement, score 2: ≥2 organs involvement) | ||||||
| Liver | − | No/unknown | Yes | − | Conjugated bilirubin >2 times upper normal limit | 1 |
| Kidney | − | No/unknown | Yes | − | 0 | |
| Lung | − | No/unknown | Yes | − | 0 | |
| Muscle/heart | − | No/unknown | Yes | − | 0 | |
| Pancreas | − | No/unknown | Yes | − | 0 | |
| Resolution ≥15 days | − | No/unknown | Yes | − | 0 | |
| Exclusion of other causes | ||||||
| (If none is positive and ≥3 of the following are negative): Antinuclear antibody, blood culture, serology for hepatitis A/hepatitis B/hepatitis C, serology for chlamydia/mycoplasma | − | − | Yes | 0 | Negative serology for hepatitis B and C, rest not done | 0 |
| Total score | 7 | |||||
Interpretation based on final score: >5: definite, 4–5: probable, 2–3: possible, <2: no case
| Criterion | Values | Phenytoin | Temozolomide |
|---|---|---|---|
| Delay from initial drug component intake to onset of reaction (index day) | Suggestive: +3 Compatible: +2 Likely: +1 Unlikely: −1 Excluded: −3 |
2 (started >6 weeks before onset) | 2 (started <2 weeks before onset) |
| Drug present in the body on index day | Definite: 0 Doubtful: 1 Excluded: 3 |
0 (continued up to index day) | 0 (continued up to index day) |
| Prechallenge/rechallenge | Positive specific for disease and drug: 4 Positive specific for disease or drug: 2 Positive unspecific: 1 Not done/unknown: 0 Negative: 2 |
0 (no known previous exposure to this drug) | 0* (no known previous exposure to this drug) |
| Dechallenge | Neutral: 0 Negative: 2 |
0 | 0 |
| Type of drug (notoriety) | Strongly associated: 3 Associated: 2 Suspected: 1 Unknown: 0 Not suspected: 1 |
3 (high risk) | 0 (no epidemiological studies, single case report) |
| Other cause | Possible: 1 | 0 (no other drug with score >3) | –1# (another drug with score >3) |
| Total score | 5 | 1 |
Interpretation of final score: <0, Very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable; *Scored 4 on reassessment after second episode as drug rash with eosinophilia and systemic symptoms syndrome recurred after re-administration of temozolomide; #Scored 0 at reassessment after second episode, as no other cause of drug rash with eosinophilia and systemic symptoms syndrome was likely
Temozolomide and phenytoin were discontinued and he was prescribed oral prednisolone 40 mg/day along with antihistamines and moisturizer. Alternate anti-epileptic drug of a different class (levetiracetam) was prescribed in lieu of phenytoin. At follow up visit after two weeks, facial oedema had subsided, cutaneous lesions were resolving with desquamation, total leucocyte count was 18,100 cells/mm3 and absolute eosinophilic count had declined to 543 cells/mm3. Liver function tests was normalised.
One month after the episode, keeping in view the essential nature of chemotherapy and “unlikely” causality as per algorithm for assessment of drug causality in epidermal necrolysis score, temozolomide was restarted at a dose of 75 mg/m2 with close monitoring, while prednisolone 30 mg/day was continued. One week after the rechallenge, the patient again developed facial oedema and maculopapular rash. Repeat investigations showed elevated leucocyte count of 14,320 cells/mm3 with lymphopenia (845 cells/mm3, reference range: 1000–3000 cells/mm). Temozolomide was immediately withdrawn and prednisolone was continued with a slow taper, along with the advice to switch to an alternative chemotherapeutic agent. His lesions resolved after the temozolomide was discontinued. Repeat algorithm for assessment of drug causality in epidermal necrolysis score was 6 for temozolomide (indicating “very probable” association), compared to 2 for levetiracetam. A timeline of events is presented in Figure 2.
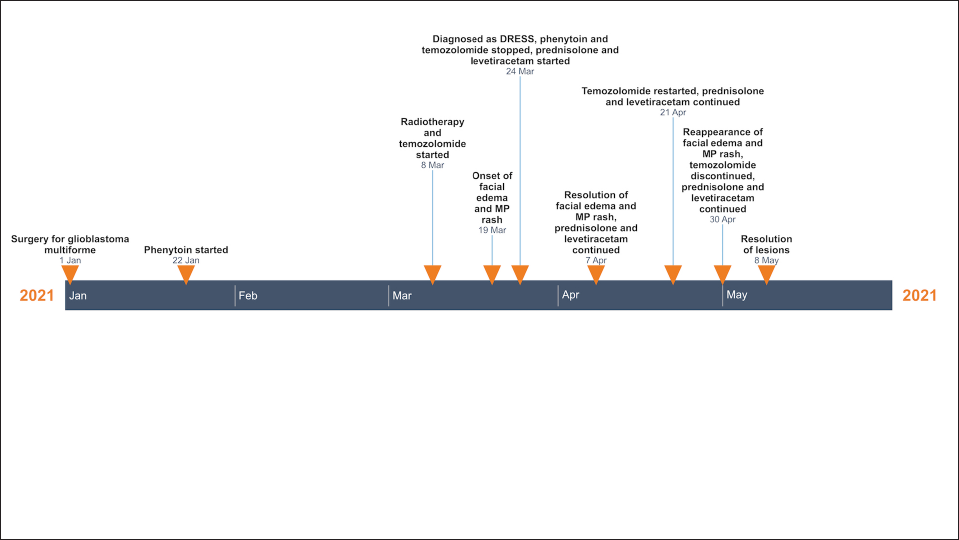
- Timeline of events in index case (DRESS: drug rash with eosinophilia and systemic symptoms, MP: maculopapular)
Temozolomide was first approved for the management of newly diagnosed glioblastoma multiforme in 2005. The dermatologic side effects with temozolomide are infrequent.2-4 Among 300 Korean patients of malignant gliomas treated with temozolomide, 5.3% developed a skin rash, none of them were severe.4 Literature search revealed a single report of temozolomide-induced DRESS syndrome.5 Likelihood of causation in previously reported case, however, cannot be commented upon, as detailed clinical history and timeline of events were not provided.
While the latency period for drug rash with eosinophilia and systemic symptoms is usually 2–6 weeks, cases occurring before two weeks have been documented in literature.6 In our initial assessment, phenytoin was considered the apposite culprit due to the compatible timeline and the drug being a well-documented cause of DRESS syndrome. The literature search conducted for assessment of drug causality revealed a single case report of DRESS syndrome following temozolomide.5 Although temozolomide appeared to be an unlikely suspect initially, rechallenge confirmed it as the culprit. Resolution of symptoms by dechallenge in both episodes further cements the culpability of temozolomide. It is imperative to have an open mind regarding drug causality in patients receiving multiple medications due to underlying comorbidities. Our case also highlights the shortcoming of drug causality scores for drugs not traditionally considered “high risk.” Awareness regarding this uncommon and potentially life-threatening adverse effect of temozolomide is crucial. Immediate withdrawal of the drug is essential for management of DRESS syndrome, with delays resulting in poorer outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Clinical, biochemical, and serologic predictors of drug reaction with eosinophilia and systemic symptoms syndrome: A prospective case-control study. J Am Acad Dermatol. 2021;85:901-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stevens-Johnson Syndrome and toxic epidermal necrolysis overlap due to oral temozolomide and cranial radiotherapy. Am J Clin Dermatol. 2009;10:264-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse drug reaction associated with oral temozolomide presenting as dermal and subcutaneous plaques and nodules. JAAD Case Rep. 2015;1:286-8.
- [CrossRef] [PubMed] [Google Scholar]
- Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J Korean Med Sci. 2014;29:980-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful desensitization with temozolomide in a patient with DRESS. Allergy. ;73:813. (Suppl. 105) abstr. 1773, Aug 2018. Available from: URL: [abstract] - Switzerland
- [Google Scholar]
- Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: A retrospective study. J Am Acad Dermatol. 2020;82:606-11.
- [CrossRef] [PubMed] [Google Scholar]





