Translate this page into:
Tender indurated plaque with ulceration on the chin
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Dipankar De
Department of Dermatology, Venereology, and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Khullar G, De D, Saikia UN, Handa S. Tender indurated plaque with ulceration on the chin. Indian J Dermatol Venereol Leprol 2014;80:275-277 |
A 35-year-old lady presented with a one-year history of an erythematous, painful lesion on the left side of the chin. It started as a pea-sized papule on the chin, which gradually increased in size to become a plaque extending upto the left submandibular area. There was history of an occasional purulent discharge. There was no history of preceding trauma, dental infection, photosensitivity, joint pains, fever or other systemic complaints. Physical examination revealed a single erythematous indurated nodulo-plaque measuring 5 × 3 cm on left side of the chin extending upto submandibular area. Overlying skin had multiple ulcers covered with yellowish-brown adherent crust and atrophic scarring at places [Figure - 1]. It was firm to hard in consistency, tender and free from underlying structures. Anti-nuclear antibody (ANA) was 1+ in a speckled pattern and anti-double stranded DNA (ds DNA) antibody was negative. On histological examination, the epidermis showed follicular plugging and basal cell vacuolization. In addition to perivascular and perifollicular lymphomononuclear infiltrate in the superficial and mid-dermis [Figure - 2]a. A lympho-histiocytic infiltrate in the fat lobules with diffuse necrosis of adipocytes was also observed [Figure - 2]b].
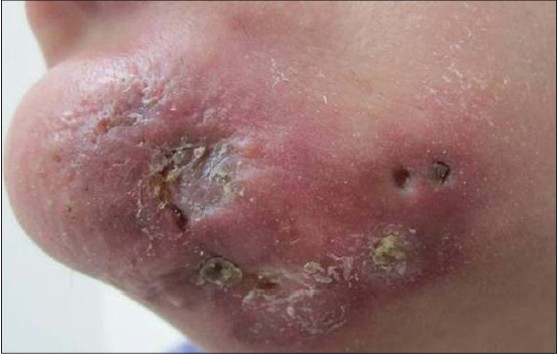 |
| Figure 1: An ill-defi ned erythematous indurated plaque measuring 5 × 3 cm with overlying skin showing adherent yellowish-brown crust over ulcerated areas on the left side of chin extending to the submandibular area |
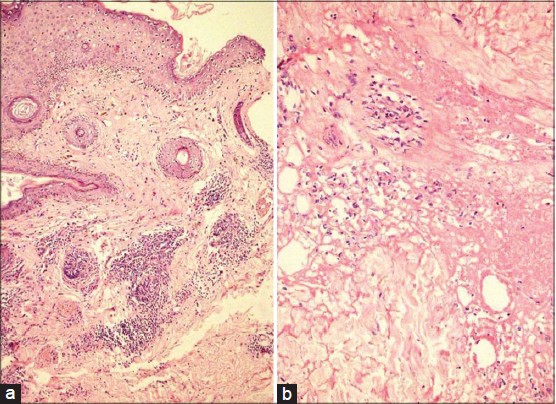 |
| Figure 2: (a) Follicular plugging, basal cell vacuolization, perivascular and perifollicular lymphomononuclear infi ltrate in superfi cial and mid-dermis (H and E, ×10) (b) Lobular panniculitis with necrosis of adipocytes (H and E, ×40) |
WHAT IS YOUR DIAGNOSIS?
Answer:
Lupus erythematosus panniculitis
DISCUSSION
Subcutaneous lesions in patients with lupus erythematosus (LE) were first described by Kaposi in 1883 and were termed lupus erythematosus of Kaposi-Irgang by Arnold in 1956. [1] LE panniculitis (LEP), a distinct variant of LE, commonly presents in the 3 rd to 6 th decade of life with female to male ratio of 2:1. The characteristic lesions consist of tender subcutaneous nodules and plaques. Ulceration is less frequent and described in about 28% cases of LEP. [2] The association of LEP with discoid LE (DLE) varies from 33% to 60%. [2],[3] Although 10-42% patients may have associated systemic lupus eythematosus (SLE), [4] LEP has been reported to occur in only 2-5% cases of SLE and is a marker of less severe forms of SLE. [5]
Histopathologically, LEP is classified as a lobular panniculitis. However, it is frequently accompanied by septal involvement; and therefore, termed as ′mixed panniculitis′. The histopathologic criteria proposed for the diagnosis of LEP include major (important for the diagnosis) and minor criteria (not necessary for diagnosis). [5] The major criteria are: (1) hyaline fat necrosis, (2) lymphocytic aggregates and lymphoid follicle formation, (3) periseptal or lobular lymphocytic panniculitis, and (4) calcification. The minor criteria are: (1) changes of DLE in the overlying skin, (2) lymphocytic vascular inflammation, (3) hyalinization of sub-epidermal zone, (4) mucin deposition, (5) histiocytes and small granulomas, and (6) infiltrates of plasma cells and eosinophils. Overlying dermo-epidermal findings of DLE may be present in 50-75% cases even without clinical evidence of DLE. Direct immunoflourescence may show granular deposition of IgG, IgM, and C3 at the dermal-epidermal junction and blood vessels of deep dermis and subcutis in 50-70% cases, [1],[5] particularly when there is concomitant DLE.
Treatment of LEP is challenging as it has a chronic and relapsing course. Antimalarials are the first line of treatment. Oral corticosteroids though beneficial, [5] should be used for severe cases with associated SLE due to their side effects. Thalidomide, dapsone, cyclosporine, intravenous immunoglobulins and rituximab have shown efficacy in intolerant or refractory cases. [6] Our patient, was started on hydroxychloroquine (HCQ) 400 mg/day. Four days after commencing HCQ, she developed a maculopapular drug rash. HCQ was stopped and she was administered oral prednisolone 40 mg/day, which was tapered by 10 mg every month. At two months, the ulceration healed and there was significant reduction in tenderness and induration of the plaque [Figure - 3]. Post-treatment skin biopsy showed normalization of basal layer with reduction in the dermal infiltrate [Figure - 4]a] and early collagenization of subcutaneous fat, suggestive of healing [Figure - 4]b].
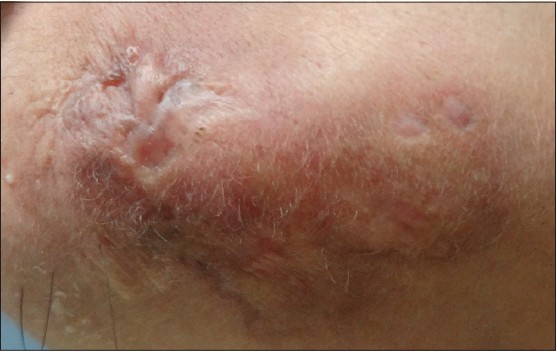 |
| Figure 3: Marked decrease in erythema and induration of plaque and healing of ulceration with atrophic scarring after 2 months of oral prednisolone |
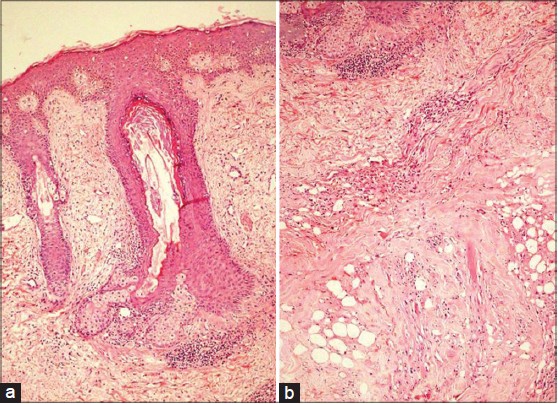 |
| Figure 4: (a) Intact basal layer with reduction in dermal inflammation (H and E, ×10) (b) Early collagenization of subcutaneous fat (H and E, ×40) |
Patients with LEP should be followed up regularly, lest they develop SLE. Repeat biopsy with immunohistochemistry and the T-cell receptor gene rearrangement studies may be required in refractory cases to rule out subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Histopathologically, LEP encompasses a spectrum of features with small, mature lymphocytes showing polyclonality on one hand and pleomorphic lymphocytes with hyperchromatic nuclei demonstrating deletion of pan T-cell markers and monoclonal T-cell receptor gene rearrangement on the other. The term subcutaneous lymphoid dyscrasia has been proposed to include atypical cases of LEP, SPTCL and indeterminate lymphocytic lobular panniculitis. [7] Further, vacuolar interface dermatitis and dermal mucinosis has been reported in SPTCL, thereby pointing to an overlap between the two entities. [8] However, Pincus et al.,[9] suggested that lymphoid follicles with reactive germinal centers and mixed infiltrate comprising of plasma cells may favor LEP over SPTCL.
| 1. |
Arnold HL Jr. Lupus erythematosus profundus; commentary and report of four more cases. AMA Arch Dermatol 1956;73:15-33.
[Google Scholar]
|
| 2. |
Martens PB, Moder KG, Ahmed I. Lupus panniculitis: Clinical perspectives from a case series. J Rheumatol 1999;26:68-72.
[Google Scholar]
|
| 3. |
Tuffanelli DL. Lupus erythematosus (panniculitis) profundus: A classic revisited commentary and report of 22 cases. Hawaii Med J 1982;41:394-7.
[Google Scholar]
|
| 4. |
Fraga J, Garcia-Diez A. Lupus erythematosus panniculitis. Dermatol Clin 2008;26:453-63.
[Google Scholar]
|
| 5. |
Peters MS, Su WP. Lupus erythematosus panniculitis. Med Clin North Am 1989;73:1113-26.
[Google Scholar]
|
| 6. |
Braunstein I, Werth VP. Update on management of connective tissue panniculitides. Dermatol Ther 2012;25:173-82.
[Google Scholar]
|
| 7. |
Magro CM, Crowson AN, Kovatich AJ, Burns F. Lupus profundus, indeterminate lymphocytic lobular panniculitis and subcutaneous T-cell lymphoma: A spectrum of subcuticular T-cell lymphoid dyscrasia. J Cutan Pathol 2001;28:235-47.
[Google Scholar]
|
| 8. |
Gonzalez EG, Selvi E, Lorenzini S, Maggio R, Mannucci S, Galeazzi M, et al. Subcutaneous panniculitis-like T-cell lymphoma misdiagnosed as lupus erythematosus panniculitis. Clin Rheumatol 2007;26:244-6.
[Google Scholar]
|
| 9. |
Pincus LB, LeBoit PE, McCalmont TH, Ricci R, Buzio C, Fox LP, et al. Subcutaneous panniculitis-like T-cell lymphoma with overlapping clinicopathologic features of lupus erythematosus: Coexistence of 2 entities? Am J Dermatopathol 2009;31:520-6.
[Google Scholar]
|
Fulltext Views
4,444
PDF downloads
3,106





