Translate this page into:
The bidirectional association between type 2 diabetes and psoriasis: Two retrospective cohort studies
2 Department of Dermatology, Chung Shan Medical University Hospital; Department of Dermatology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
3 College of Medicine; Department of Health Services Administration, China Medical University, Taichung, Taiwan
4 Department of Internal Medicine, Division of Endocrinology and Metabolism, National Taiwan University Hospital Hsin-Chu Branch, Hsinchu, Taiwan
5 Department of Health Services Administration, China Medical University; Department of Food Nutrition and Health Biotechnology, Asia University, Taichung, Taiwan
Correspondence Address:
Fung-Chang Sung
Department of Health Services Administration, China Medical University, 91 Hseuh Shih Road, Taichung 404
Taiwan
| How to cite this article: Chiu HY, Hung CJ, Muo CH, Fan KC, Sung FC. The bidirectional association between type 2 diabetes and psoriasis: Two retrospective cohort studies. Indian J Dermatol Venereol Leprol 2020;86:366-374 |
Abstract
Background: Inflammation plays a crucial role in both type 2 diabetes mellitus (T2DM) and psoriasis pathogenesis; thus, a bidirectional association between them is likely suspected.
Aims: We investigated the possible bidirectional association between T2DM and psoriasis.
Methods: Using the Taiwan National Health Insurance Research Database, we conducted two retrospective cohort studies. The analysis of psoriasis onset in relation to T2DM status included 31,697 patients with diabetes and 126,788 nondiabetic control subjects (Analysis 1). The analysis of T2DM onset in relation to psoriasis status included 1,947 psoriatic patients and 7,788 nonpsoriatic control subjects (Analysis 2). The follow-up period was from 2000 to the date of the outcome of interest, date of death, or December 31, 2013. Cox proportional models were used to estimate the relative hazards.
Results: In Analysis 1, Kaplan–Meier (KM)-based cumulative incidence of psoriasis was higher in the T2DM cohort than that in the non-T2DM cohort (1.2% vs. 0.7%). The covariate-adjusted hazard ratio (HR) was 1.40 [95% confidence interval (CI), 1.20–1.63] for patients with T2DM. Analysis 2 revealed KM-based cumulative T2DM incidences of 18.7% and 13.1% in psoriatic and nonpsoriatic subjects, respectively. The adjusted HR for incident T2DM was higher in patients with psoriasis (1.38; 95% CI, 1.20–1.58).
Limitation: This article may not represent the population worldwide and patient selection bias may exist.
Conclusion: Our results provide evidence for a bidirectional T2DM–psoriasis association. T2DM and psoriasis are common worldwide; thus, our findings have public health implications for the early identification and management of these comorbid diseases.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by insulin resistance and impaired islet beta cell function.[1] Psoriasis is a chronic immune-mediated inflammatory disorder that affects 0.1%–3.0% of adults worldwide.[2],[3],[4] T2DM and psoriasis are among the most prevalent diseases and are associated with a number of comorbidities.[5],[6],[7],[8] The rapid worldwide increase in the prevalence of T2DM and psoriasis has had substantial adverse effects on healthcare systems and affected patients.[1],[9]
Accumulating evidence suggests that inflammation has a crucial intermediary role in T2DM development and progression.[10] Inflammation is also thought to be a key component in the pathogenic mechanism of psoriasis.[2],[11] Inflammatory mediators such as C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α are frequently elevated in patients with T2DM, as well as in patients with psoriasis.[10],[11],[12],[13],[14],[15] Moreover, subclinical inflammation in psoriasis can trigger psoriatic disease recurrence.[16] These findings suggest that T2DM and psoriasis share an inflammatory mechanism and may be linked.
Some studies have reported that diabetes was prevalent among patients with psoriasis.[17],[18],[19] However, the underlying link between psoriasis and diabetes is not well understood. Studies examining whether persons with diabetes have an elevated risk of psoriasis have been limited in scope and have yielded inconclusive findings.[10],[20],[21] A recent study found that T2DM increased the risk of developing rheumatoid arthritis (RA), which, like psoriasis, is an immune-mediated chronic inflammatory disease.[22]
We hypothesized that chronic low-grade inflammation in T2DM may trigger psoriasis development in genetically susceptible individuals and, conversely, that persons with psoriasis have a higher risk of subsequently developing T2DM. This study investigated the potential bidirectional association between T2DM and psoriasis in a large, nationally representative, population-based cohort of predominantly Chinese patients in Taiwan.
Methods
Data source
This retrospective cohort study used data from the Taiwan Longitudinal Health Insurance Database (LHID) 2000, which is a subset of the National Health Insurance Research Database (NHIRD). NHIRD data are compiled from the Taiwan National Health Insurance (NHI) system, which was launched in 1995 to finance healthcare for all citizens. The NHI provides care for approximately 99.5% of the 23.74 million citizens of Taiwan. In LHID 2000, approximately 1,000,000 representative individuals were randomly sampled from all those in NHIRD in 2000. The database comprises comprehensive information on insured subjects, such as demographic data, dates of clinical visits, diagnostic codes, prescription details, and expenditures. A multistage stratified systematic sampling design was used and there were no statistically significant differences in sex, age, or average insured payroll-related amount between the LHID sample and the entire NHIRD population (http://nhird.nhri.org.tw/en/Data_Subsets.html). All subject information was anonymized and deidentified to protect privacy. The study protocol was approved by the local investigational research bureau of National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan (103-024-E).
Study subjects
In the first analysis, we identified patients who received a new diagnosis of T2DM and those without a T2DM diagnosis (control group). We randomly selected four control subjects without a T2DM diagnosis per patient with psoriasis, matched for age, sex, and index date from LHID 2000. A T2DM diagnosis was defined as at least two ambulatory claims or at least one inpatient claim with International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes of 250.xx, except for type 1 diabetes (ICD-9-CM codes 250.01, 250.11, 250.13, 250.21, 250.23, 250.31, 250.33, 250.41, 250.43, 250.51, 250.53, 250.61, 250.63, 250.71, 250.73, 250.81, 250.83, 250.81, and 250.93), between 2000 and 2004. In the second analysis, we selected individuals with a new psoriasis diagnosis (ICD-9-CM codes 696.0, 696.1, and 696.8) and those without a psoriasis diagnosis as the control group. For each psoriasis patient, four subjects without psoriasis, matched for age, sex, and index date, were randomly selected from LHID 2000. To ensure the accuracy of the psoriasis diagnoses, subjects were selected only if they had received at least two diagnoses of psoriasis by dermatologists during ambulatory visits or inpatient care between 2000 and 2004. The dates of newly diagnosed T2DM (in Analysis 1) and psoriasis (in Analysis 2) were designated as the index dates from which follow-up began. To ascertain the temporal association between the risk factor and the subsequent development of outcome, we had excluded cases with outcome at enrolment. In Analysis 1, we excluded patients who had received a psoriasis diagnosis before a T2DM diagnosis. Similarly, patients with a T2DM diagnosis before a psoriasis diagnosis were excluded from Analysis 2.
Outcome and relevant variables
All subjects were followed from the index date until the incidence of psoriasis (Analysis 1) or T2DM (Analysis 2), until they were censored at the end of the study period (December 31, 2013), or until the date of disenrollment (usually because of death). The primary outcome was the first ambulatory visit or hospitalization for any psoriasis (Analysis 1) or T2DM (Analysis 2), regardless of whether the patient survived or died after the outcome event.
We adjusted for potential confounders by including medical history and medication use in the preceding year. The medical history included hypertension (ICD-9-CM codes 401–405), hepatitis C (070.41, 070.44, 070.51, 070.54, and V02.62), depression (296.2, 296.3, 300.4, 309.1, and 311), coronary artery disease (410–414), hyperlipidemia (272), obesity (278), gout (274), chronic kidney disease (403, 404, 582, 583, 585, 586, 588, V42.0, V45.1, and V56), alcoholic liver disease (571.0, 571.1, 571.2, and 571.3), and chronic obstructive pulmonary disease (490–496). Prior medication use included statins, steroids, diuretics, β-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, interferon, lithium, and antipsychotic drugs.
Statistical analysis
Intergroup differences were evaluated by independent-samples t-tests for continuous variables and Chi-square or Fisher's exact tests for categorical variables. In Analysis 1, we calculated the incidence density (cases per 10,000 person-years) of psoriasis stratified by sex, age, and comorbidities. Cox regression models were used to estimate the T2DM cohort to non-T2DM cohort hazard ratio (HR) of psoriasis development. The multivariable model including the variables of age, sex, degree of urbanization, income group, comorbidities, medications used, and number of outpatient visits in the first year after the index date was used to estimate the adjusted HR (aHR). The cumulative incidence of psoriasis was computed using Kaplan–Meier (KM) method, and differences between two cohorts were examined by log-rank tests. Similar data analysis procedures were used for Analysis 2. We also conducted a test for interaction to evaluate for statistically significant differences in subgroups. If the P value was significant, we concluded that the effect of T2DM/psoriasis on the outcome differed within the subgroups. The data were managed and statistical analysis was performed using SAS Version 9.3 (SAS Institute, Cary, NC, USA). A two-tailed P value of less than 0.05 was considered to indicate statistical significance.
Results
Analysis 1: Cohort analysis of the psoriasis risk in relation to diabetes status
During 2000–2004, we identified 31,697 diabetic patients and 126,788 matched control subjects. The distributions of age and sex were similar between the cohorts. The mean age was approximately 55 years, and most patients in both groups were male. Patients with T2DM were more likely than the matched control subjects to have comorbidities including hypertension, coronary artery disease, cerebrovascular disease, depression, hyperlipidemia, hepatitis C, obesity, gout, chronic obstructive pulmonary disease, chronic kidney disease, and alcoholic liver disease (all P < 0.001) [Table - 1].
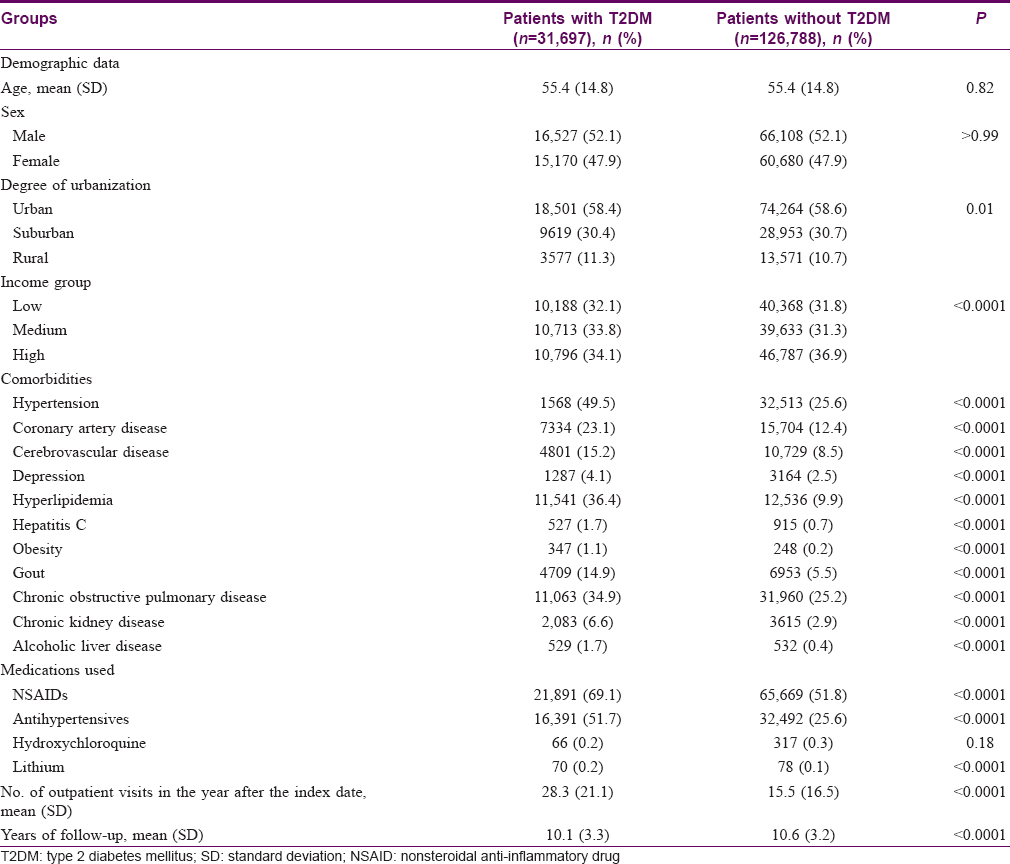
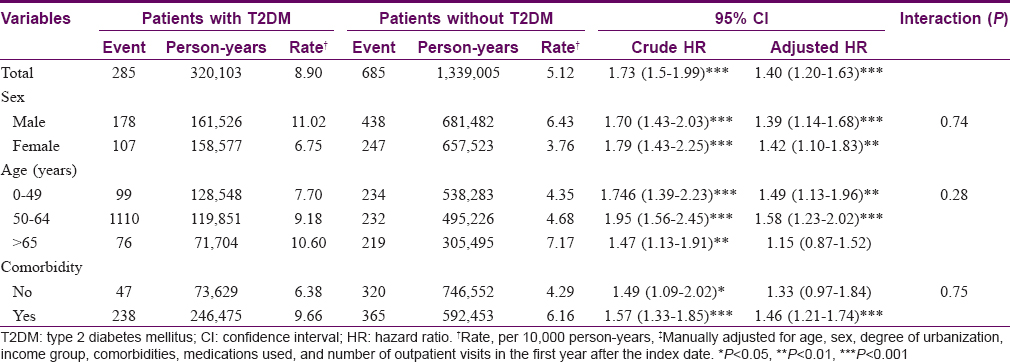
The median follow-up periods were 10.1 years in the diabetic group and 10.6 years in the nondiabetic control group. The incidence of psoriasis was higher in the diabetic cohort than that in the nondiabetic cohort, with KM-based cumulative incidences of 1.2% and 0.7%, respectively [Figure - 1]. The diabetic cohort remained associated with a significantly higher risk for psoriasis after adjusting for age, sex, degree of urbanization, income group, comorbidities, medications used, and number of outpatient visits in the first year after the index date [aHR 1.40; 95% confidence interval (CI), 1.20–1.63; [Table - 2]. The aHRs for psoriasis were significantly higher in both sexes. In subgroup analysis, although the interaction P value was not statistically significant, the aHR among diabetic patients was highest for patients age 50–64 years (1.58; 95% CI, 1.23–2.20), and the aHR for psoriasis was higher in diabetic patients with comorbidities than that in those without comorbidities [Table - 2].
 |
| Figure 1: Cumulative incidence of psoriasis in type 2 diabetes and control cohorts (general population without diabetes) |
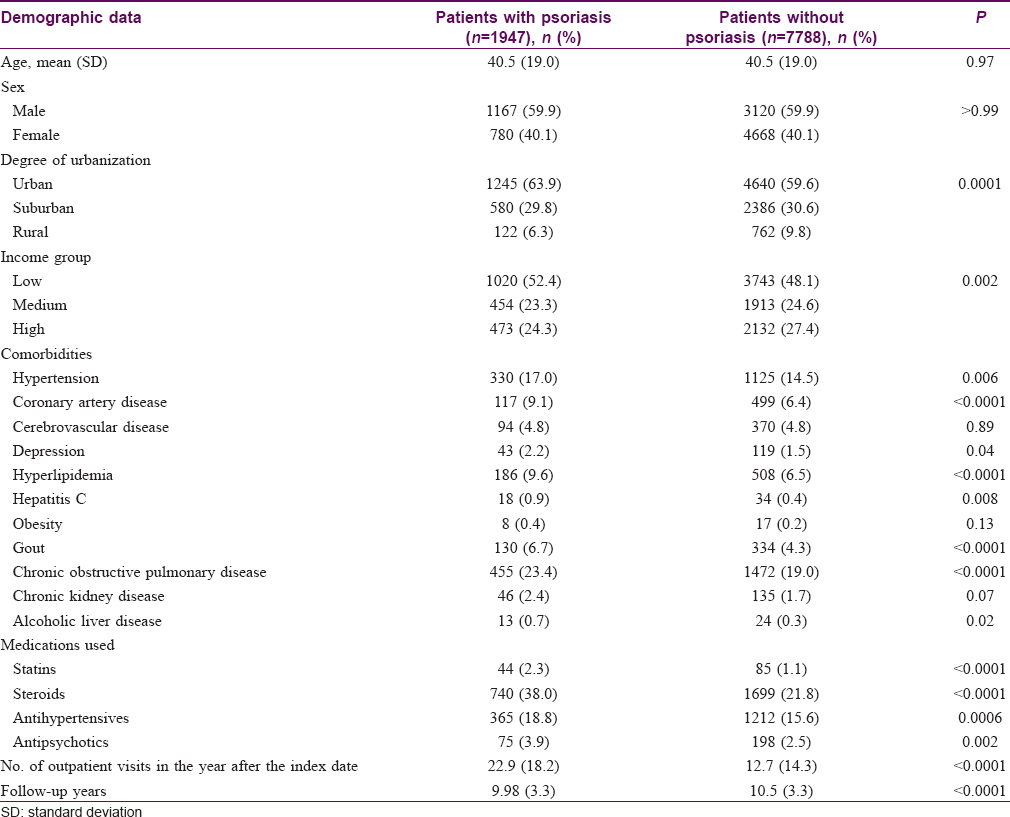
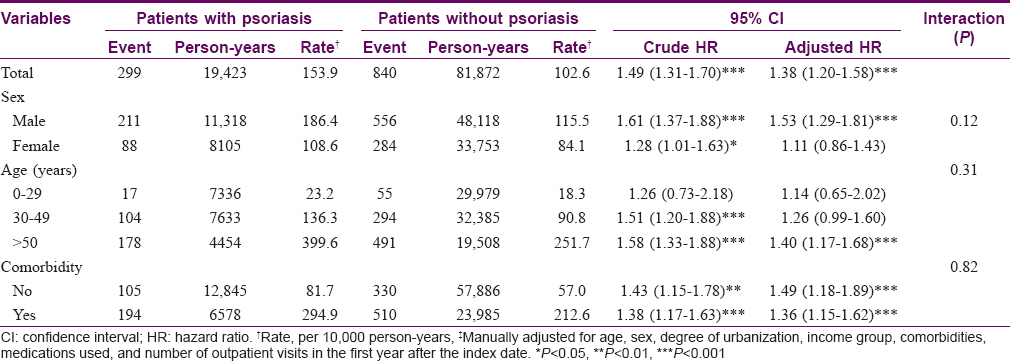
Analysis 2: Cohort analysis of diabetes risk in relation to psoriasis status
The distributions of baseline characteristics in the psoriasis and nonpsoriasis cohorts are summarized in [Table - 3]. Patients with psoriasis had lower incomes and more comorbidities than those in the nonpsoriasis group. The KM-based cumulative incidence of T2DM was 18.7% in patients with psoriasis and 13.1% in controls during mean follow-up periods of 9.98 and 10.5 years, respectively [Figure - 2].
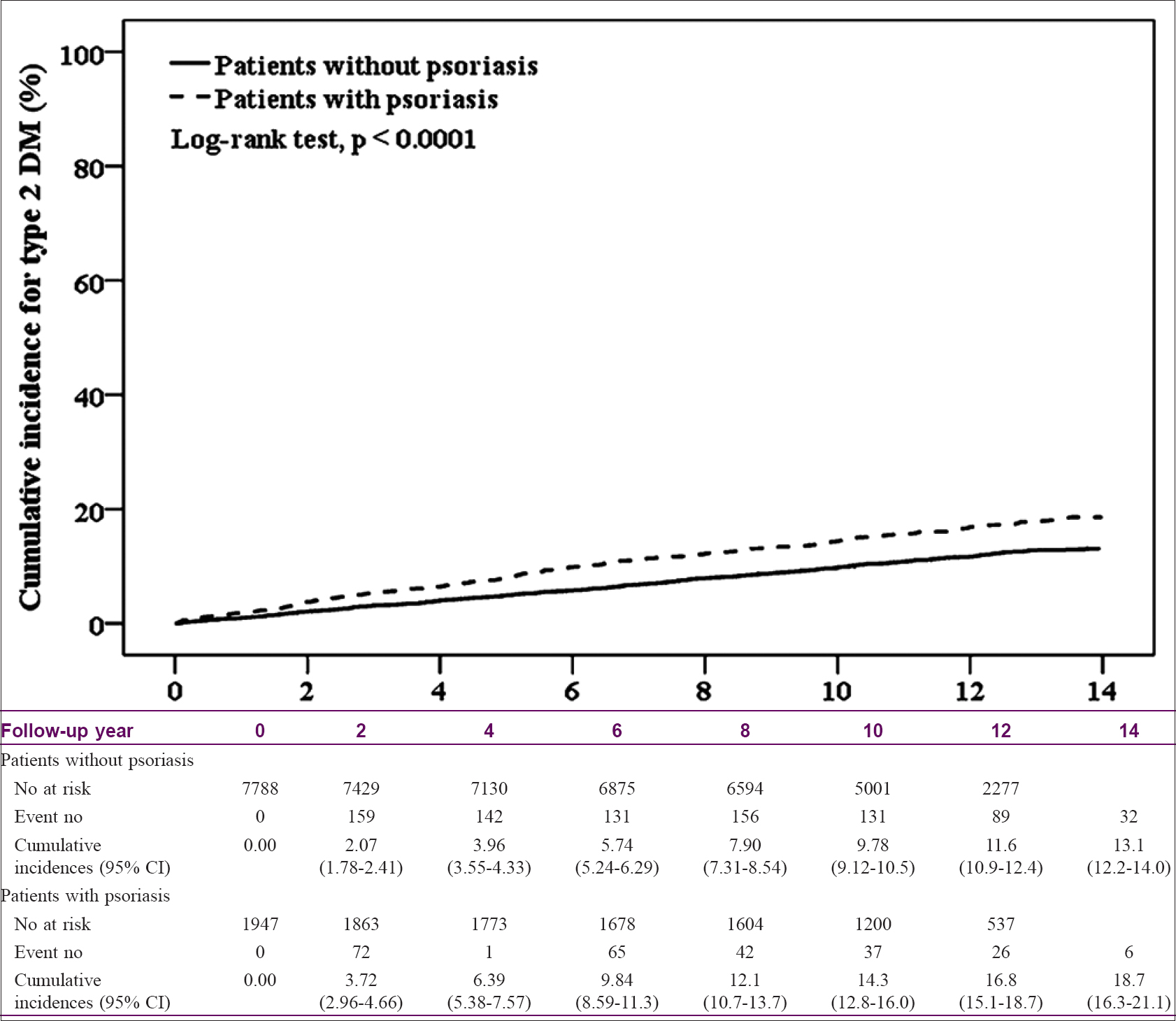 |
| Figure 2: Cumulative incidence of type 2 diabetes in the psoriasis and control cohorts (general population without psoriasis) |
After adjusting for age, sex, degree of urbanization, income group, comorbidities, medication use in the previous year, and number of outpatient visits in the first year after the index date, the aHR for diabetes during the 10-year follow-up period for subjects with psoriasis was 1.38 (95% CI, 1.20–1.58) when compared with nonpsoriatic subjects [Table - 4]. Among patients with psoriasis, although the interaction P value was not statistically significant, the risk of T2DM was higher for men than that for women. In age-stratified analysis, the risk of diabetes after a psoriasis diagnosis was highest for patients age 50 years or older (aHR, 1.40; 95% CI, 1.17–1.68) [Table - 4].
Discussion
Analysis 1: T2DM and subsequent psoriasis risk
The relationship between diabetes and psoriasis is likely to be complex. Unfortunately, few studies have investigated the risk of psoriasis in patients with T2DM. Recent research indicates that T2DM increases blood levels of inflammatory markers such as TNF-α and IL-6.[10],[12] Elevations of these inflammatory markers may be pathogenetic factors in psoriasis.[11],[13],[23] Moreover, hyperglycemia has been reported to drive secretion of several proinflammatory cytokines, including TNF-α, IL-1 β, IL-6, and IL-22, by macrophages and T cells in adipose tissue, which have also been implicated in psoriasis pathogenesis.[2],[4],[14],[24],[25] Elevated circulating glucose concentrations activate the NLRP3 inflammasome, which may play a pivotal role in psoriasis susceptibility.[10],[26] Furthermore, increases in Th17 and CD8+ T cells in both T2DM and psoriasis may explain the link between these diseases.[27],[28] Emerging evidence suggests that the chronic inflammatory state of T2DM could induce release of cryptic “self” antigens upon destruction of inflammation-induced tissue, thereby leading to autoimmune activation.[27],[29] This hypothesis is further supported by a recent nationwide, population-based, case–control study that showed a 3.6-fold increased risk of developing RA in patients age 20–44 years with T2DM than that in those without T2DM.[22] The increased risk of the autoimmune disorder RA in patients with T2DM might be attributable to chronic inflammation in T2DM.[22]
Existing evidence indicates that psoriasis and diabetes have a common genetic background. Genetic variations in T-lymphocyte antigen 4, Toll-like receptor 3, IL-12B, IL-23R, and IL-23A increase the risks of both diseases.[30],[31] Wolf et al. reported that psoriasis is associated with pleiotropic susceptibility loci identified in T2DM.[32] The present findings showed that the psoriasis risk was 40% higher in patients with T2DM than that in individuals without T2DM after adjusting for confounding variables.
Analysis 2: Psoriasis and subsequent T2DM risk
Several observational studies reported increased prevalence and incidence of T2DM among patients with psoriasis,[18],[19],[33] although several studies found no association between psoriasis and diabetes.[34],[35],[36] Systemic inflammation is a potential mechanistic link between these diseases.[18],[19] Chronic inflammation is detrimental to β-cell function and may increase insulin resistance, which commonly precedes the development of T2DM.[22],[37] A meta-analysis of 10 prospective studies that evaluated data from 19,709 participants showed that elevated levels of CRP and IL-6 significantly increased the risk of T2DM.[12],[22] Elevated CRP and IL-6 levels were also observed in patients with psoriasis.[11],[15] One study reported that psoriatic keratinocytes release proinflammatory cytokines, including IL-1β, that induce insulin resistance and may favor the development of diabetes.[28] Moreover, the use of salsalate to modulate inflammatory reactions in patients with T2DM improved glycemic control, which suggests that inflammation contributes to insulin resistance and diabetes development.[22] Our results showing that subjects with psoriasis have a higher risk of developing T2DM are consistent with these earlier findings. Although the incidence of diabetes among psoriasis cases seemed higher than that of psoriasis among diabetes subjects, the aHR for diabetes among psoriasis cases was 1.38 (95% CI, 1.20–1.58), which was close to that for psoriasis among T2DM cases (adjusted HR 1.40; 95% CI, 1.20–1.63).
Strengths and limitations
Our study has several limitations. First, as is the case for other epidemiologic studies, the identification of exposure and outcome were based on claims data. To increase the validity and accuracy of the diagnoses, we selected only subjects with repeated coding, thereby minimizing this bias. Furthermore, the NHI of Taiwan has established an ad hoc committee to monitor the accuracy of claims data, prevent violations, and verify the accuracy of medical records.[38] The Bureau of NHI of Taiwan imposes heavy fines for false claims, overcharging, or malpractice for fraudulent claims. However, it was possible that the diagnosis of diabetes or psoriasis was not made in patients who did not report the disease symptoms and signs or did not visit medical facilities; thus, they might have been misclassified as control subjects. Second, more frequent hospital visits by psoriasis or diabetic patients, when compared with those in the general population, could result in potential surveillance bias. To address that issue, the multivariable regression model was further adjusted for the frequency of ambulatory care visits. The results were consistent with those of the primary models, which indicated that increased healthcare consumption and attendant surveillance bias were unlikely to contribute significantly to the results. Third, the NHIRD does not provide detailed information on smoking or drinking habits, physical activity, body mass index, dietary preferences, laboratory data, or family history, although these are potential confounding factors. However, our data analysis used the comorbidity variables of chronic obstructive pulmonary disease, alcoholic liver disease, obesity, and sociodemographic status as part of the controlling variables to substitute these unmeasured confounders. Fourth, based on the ICD-9-CM codes for psoriasis diagnosis (696.0, 696.1, and 696.8) used in this study, pustular psoriasis or arthropathic psoriasis could not be completely ruled out, though most included patients had psoriasis vulgaris.
Conclusion
The results of this study show the bidirectional association between T2DM and psoriasis. T2DM and psoriasis are more than coincidental comorbidities: T2DM increases the risk of subsequent psoriasis and psoriasis onset increases the risk of T2DM. Given the corresponding healthcare burden and adverse clinical outcomes of these medical conditions, public health and medical professionals need to be informed of the bidirectional association between T2DM and psoriasis. Knowledge of this relationship will assist in early identification and prompt management strategies which could increase the quality of life and improve outcomes of concomitant psoriasis and T2DM among patients with diabetes and psoriasis, respectively.
Declaration of patient consent
The authors used Taiwan National Health Insurance Research Database, which had encrypted the names of patients, health care providers, and medical institutions with unique and anonymous identifiers, to conduct this study. Thus, the waiver of consent from each patient and the study protocol were approved by the local investigational research bureau of National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan (103-024-E).
Financial support and sponsorship
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004), the National Taiwan University Hospital Hsin-Chu Branch (108-HCH002), the Ministry of Science and Technology, Taiwan (formerly the National Science Council; grant numbers MOST 107-2314-B-002-259-MY3), China Medical University Hospital, the Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), and the NRPB Stroke Clinical Trial Consortium (MOHW106-TDU-B-212-113004).
Conflicts of interest
There are no conflicts of interest.
| 1. |
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011;378:31-40.
[Google Scholar]
|
| 2. |
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496-509.
[Google Scholar]
|
| 3. |
Chiu HY, Huang PY, Jee SH, Hu CY, Chou CT, Chang YT, et al. HLA polymorphism among chinese patients with chronic plaque psoriasis: Subgroup analysis. Br J Dermatol 2012;166:288-97.
[Google Scholar]
|
| 4. |
Boehncke WH, Schön MP. Psoriasis. Lancet 2015;386:983-94.
[Google Scholar]
|
| 5. |
Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GA. Comorbidity in patients with diabetes mellitus: Impact on medical health care utilization. BMC Health Serv Res 2006;6:84.
[Google Scholar]
|
| 6. |
American Diabetes Association. 3 Comprehensive medical evaluation and assessment of comorbidities. Diabetes Care 2017;40:S25-32.
[Google Scholar]
|
| 7. |
Chiu HY, Chang WL, Huang WF, Wen YW, Tsai YW, Tsai TF. Increased risk of arrhythmia in patients with psoriatic disease: A nationwide population-based matched cohort study. J Am Acad Dermatol 2015;73:429-38.
[Google Scholar]
|
| 8. |
Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol 2010;130:1785-96.
[Google Scholar]
|
| 9. |
Wang TS, Hsieh CF, Tsai TF. Epidemiology of psoriatic disease and current treatment patterns from 2003 to 2013: A nationwide, population-based observational study in Taiwan. J Dermatol Sci 2016;84:340-5.
[Google Scholar]
|
| 10. |
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98-107.
[Google Scholar]
|
| 11. |
Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br J Dermatol 2013;169:266-82.
[Google Scholar]
|
| 12. |
Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013;36:166-75.
[Google Scholar]
|
| 13. |
Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005;2005:273-9.
[Google Scholar]
|
| 14. |
Chiu HY, Cheng YP, Tsai TF. T helper type 17 in psoriasis: From basic immunology to clinical practice. Dermatol Sin2012;30:136-41.
[Google Scholar]
|
| 15. |
Blauvelt A. IL-6 differs from TNF-α: Unpredicted clinical effects caused by IL-6 blockade in psoriasis. J Invest Dermatol 2017;137:541-2.
[Google Scholar]
|
| 16. |
Girolomoni G, Griffiths CE, Krueger J, Nestle FO, Nicolas JF, Prinz JC, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: A hypothesis paper. J Dermatolog Treat 2015;26:103-12.
[Google Scholar]
|
| 17. |
Khalid U, Hansen PR, Gislason GH, Lindhardsen J, Kristensen SL, Winther SA, et al. Psoriasis and new-onset diabetes: A Danish nationwide cohort study. Diabetes Care 2013;36:2402-7.
[Google Scholar]
|
| 18. |
Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol 2013;149:84-91.
[Google Scholar]
|
| 19. |
Coto-Segura P, Eiris-Salvado N, González-Lara L, Queiro-Silva R, Martinez-Camblor P, Maldonado-Seral C, et al. Psoriasis, psoriatic arthritis and type 2 diabetes mellitus: A systematic review and meta-analysis. Br J Dermatol 2013;169:783-93.
[Google Scholar]
|
| 20. |
Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286-92.
[Google Scholar]
|
| 21. |
Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based european prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes 2003;52:812-7.
[Google Scholar]
|
| 22. |
Luft VC, Schmidt MI, Pankow JS, Couper D, Ballantyne CM, Young JH, et al. Chronic inflammation role in the obesity-diabetes association: A case-cohort study. Diabetol Metab Syndr 2013;5:31.
[Google Scholar]
|
| 23. |
Chiu HY, Lo PC, Huang WF, Tsai YW, Tsai TF. Increased risk of aortic aneurysm (AA) in relation to the severity of psoriasis: A national population-based matched-cohort study. J Am Acad Dermatol 2016;75:747-54.
[Google Scholar]
|
| 24. |
Kumar P, Natarajan K, Shanmugam N. High glucose driven expression of pro-inflammatory cytokine and chemokine genes in lymphocytes: Molecular mechanisms of IL-17 family gene expression. Cell Signal 2014;26:528-39.
[Google Scholar]
|
| 25. |
Dalmas E, Venteclef N, Caer C, Poitou C, Cremer I, Aron-Wisnewsky J, et al. Tcell-derived IL-22 amplifies IL-1 β-driven inflammation in human adipose tissue: Relevance to obesity and type 2 diabetes. Diabetes 2014;63:1966-77.
[Google Scholar]
|
| 26. |
Carlström M, Ekman AK, Petersson S, Söderkvist P, Enerbäck C. Genetic support for the role of the NLRP3 inflammasome in psoriasis susceptibility. Exp Dermatol 2012;21:932-7.
[Google Scholar]
|
| 27. |
Itariu BK, Stulnig TM. Autoimmune aspects of type 2 diabetes mellitus-a mini-review. Gerontology 2014;60:189-96.
[Google Scholar]
|
| 28. |
Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 2012;9:302-9.
[Google Scholar]
|
| 29. |
Kamradt T, Mitchison NA. Tolerance and autoimmunity. N Engl J Med 2001;344:655-64.
[Google Scholar]
|
| 30. |
Suárez-Fariñas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol 2012;132:2552-64.
[Google Scholar]
|
| 31. |
Sepehri Z, Kiani Z, Javadian F, Akbar Nasiri A, Kohan F, Sepehrikia S, et al. TLR3 and its roles in the pathogenesis of type 2 diabetes. Cell Mol Biol (Noisy-le-grand) 2015;61:46-50.
[Google Scholar]
|
| 32. |
Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and crohn disease. J Med Genet 2008;45:114-6.
[Google Scholar]
|
| 33. |
Armesto S, Santos-Juanes J, Galache-Osuna C, Martinez-Camblor P, Coto E, Coto-Segura P. Psoriasis and type 2 diabetes risk among psoriatic patients in a Spanish population. Australas J Dermatol 2012;53:128-30.
[Google Scholar]
|
| 34. |
Inerot A, Enerbäck C, Enlund F, Martinsson T, Samuelsson L, Wahlström J, et al. Collecting a set of psoriasis family material through a patient organisation; clinical characterisation and presence of additional disorders. BMC Dermatol 2005;5:10.
[Google Scholar]
|
| 35. |
Kondratiouk S, Udaltsova N, Klatsky AL. Associations of psoriatic arthritis and cardiovascular conditions in a large population. Perm J 2008;12:4-8.
[Google Scholar]
|
| 36. |
Reynoso-von Drateln C, Martínez-Abundis E, Balcázar-Muñoz BR, Bustos-Saldaña R, González-Ortiz M. Lipid profile, insulin secretion, and insulin sensitivity in psoriasis. J Am Acad Dermatol 2003;48:882-5.
[Google Scholar]
|
| 37. |
Cefalu WT. Inflammation, insulin resistance, and type 2 diabetes: Back to the future? Diabetes 2009;58:307-8.
[Google Scholar]
|
| 38. |
Lu MC, Guo HR, Lin MC, Livneh H, Lai NS, Tsai TY. Bidirectional associations between rheumatoid arthritis and depression: A nationwide longitudinal study. Sci Rep 2016;6:20647.
[Google Scholar]
|
Fulltext Views
3,609
PDF downloads
1,249





