Translate this page into:
The effect of statins on severity of psoriasis: A systematic review
2 Imperial College Healthcare NHS Trust, London, UK
Correspondence Address:
Ravi Ramessur
University College Hospital, 235 Euston Rd, London NW1 2BU
UK
| How to cite this article: Ramessur R, Gill D. The effect of statins on severity of psoriasis: A systematic review. Indian J Dermatol Venereol Leprol 2017;83:154-161 |
Abstract
Background: Psoriasis is becoming increasingly recognized as a chronic systemic inflammatory disease. Statins are generally well-tolerated drugs with pleiotropic effects including decreasing inflammation and may have the potential to reduce psoriasis severity.Aims: To examine whether oral statins reduce the severity of psoriatic skin disease.
Methods: We searched MEDLINE, EMBASE and adapted for Google Scholar, Cochrane Central Register for Controlled Trials and Clinical trials.gov to January 6, 2016. We primarily examined randomized controlled trials that assessed the change in PASI score over a follow-up period of at least 8 weeks, for participants with an established diagnosis of psoriasis taking an oral statin versus placebo or other active treatment. Beyond this, we also examined other interventional studies that investigated the effect of statins on psoriasis severity using other designs. We extracted efficacy and adverse event data. The two study authors examined issues of study quality and study inclusion independently.
Results: Three studies were identified which measured the change in psoriasis severity using PASI, comparing statin with placebo or standard therapy alone in a prospective, randomized study design; these showed conflicting results. However, among the excluded studies, majority of which used a single arm, non-placebo controlled study design, most showed an improvement in PASI scores after statin use.
Limitations: Included studies were of limited sample size and quality. They were not amenable to pooled analysis.
Conclusions: This review highlights the paucity of high quality, randomized, double-blinded, placebo-controlled trials investigating the effects of statins on psoriasis severity using clinically objective measures. There is insufficient evidence that the use of oral statins as an adjunctive therapy can reduce the severity of psoriasis.
Introduction
Psoriasis is a chronic inflammatory disease of the skin, scalp, nails and sometimes joints that affects 1–2% of the general population.[1],[2] It is associated with a significant physical and psychological morbidity and is becoming increasingly recognized as a systemic inflammatory disease.[3]
The mainstay of psoriasis treatment involves topical agents (e.g., vitamin D analogs and corticosteroids) for milder disease and systemic therapy (e.g., oral immunosuppressants, phototherapy, or biological agents) for more severe disease.[4] The latter are effective, but their long-term use is often limited by potential toxicity.
In clinical terms, improvement in psoriasis management leads to a reduction in cutaneous lesions which can be objectively measured using the PASI score. This is a numerical score of the extent and activity of a patient's psoriasis (ranging from 0 [no disease] to 72 [most severe disease]) and is calculated by a reproducible formula based on the surface area and cutaneous features of the disease.[5]
Statins are a class of reversible competitive inhibitors of 3-hydroxyl-3-methylyglutaryl-coenzyme A reductase known for their cholesterol lowering effects.[6] 3-hydroxyl-3-methylyglutaryl-coenzyme A reductase is the rate-limiting step in cholesterol biosynthesis. Its inhibition thereby increases the synthesis of low-density lipoprotein receptors leading to an increased clearance of plasma low-density lipoprotein cholesterol. Statins are generally well-tolerated drugs used for prevention of atherosclerosis and cardiovascular events.
Statins have also been found to exert pleiotropic effects and decrease inflammation, atherogenesis and cardiovascular morbidity.[7] This is associated with a reduction in the release of C-reactive peptide, chemokines, cytokines and adhesion molecules, as well as modulation of T-cell activity.[8] Based on their immunomodulatory properties, statins may have the potential to attenuate the inflammatory component of psoriatic disease.
Methods
Objective
The objective of this work is to explore whether oral statins improved the severity of psoriatic skin disease.
P-participants aged over 18 and over with psoriasis, I-statin therapy, C-placebo or other active treatment, O-change in Psoriasis Area and Severity Index score.
Protocol and registration
Methods of the analysis and inclusion criteria were specified in advance as detailed below and registered on PROSPERO (http://www.crd.york.ac.uk/PROSPERO/).
Eligibility studies
- Types of participants: Trials including participants over age 18 with an established diagnosis of psoriasis were considered
- Types of intervention: Trials involving the initiation of an oral statin in any dose to statin-naive participants compared with placebo or other active treatment were considered
- Types of outcome: Studies had to include change in Psoriasis Area and Severity Index score over a follow-up period of at least 8 weeks as either a primary or secondary outcome.
Information sources
Studies were identified by searching electronic databases and scanning reference lists of articles. No limits were applied for language and foreign papers were translated using the Google Translate function. A translator confirmed the accuracy of translation and also assisted with any areas that required clarification. This search was applied to MEDLINE (1946 - January 6, 2016), EMBASE (1947 - January 6, 2016) and adapted for Google Scholar (January 6, 2016). Adapted searches [Appendix 1] [SUPPORTING:1] for the Cochrane Central Register for Controlled Trials and Clinical trials.gov databases were also performed to January 6, 2016. We searched reference lists of retrieved articles and relevant reviews to identify potential additional eligible studies.
Search
We used the following search terms to search all trials registers and databases [Appendix 2] [SUPPORTING:2]: statin, statins, simvastatin, atorvastatin, pravastatin, rosuvastatin, fluvastatin, psoriasis, randomized controlled trial, randomized controlled trials, controlled clinical trial, random allocation, double-blind method, single-blind method, clinical trials, clinical trial, placebos, placebo, random, research design, comparative study, evaluation studies, follow-up studies and prospective studies.
Study selection
We determined eligibility by reading the title and abstract of each study identified in the search. We eliminated studies that clearly did not satisfy the inclusion criteria and obtained full copies of the remaining studies. The two authors read these studies independently and reached agreement by discussion in any cases of discrepancy. The studies were not anonymized in any way before assessment. A summary of the study selection process is shown in [Figure - 1].
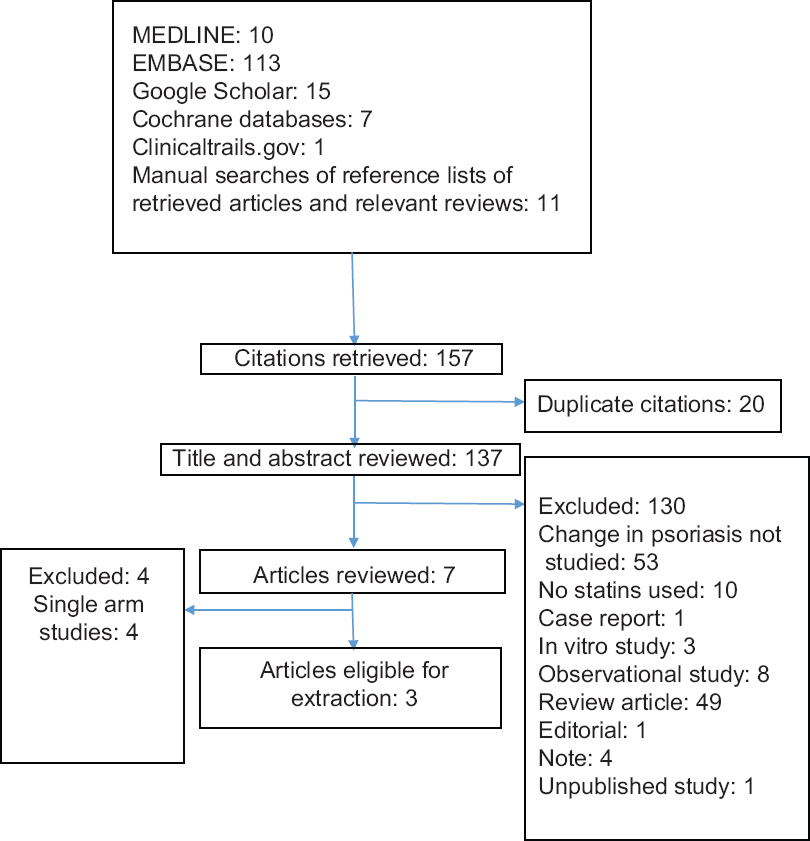 |
| Figure 1: A flowchart summarizing the study selection process |
Data extraction and management
The two review authors extracted and agreed on data, using a standard form, before any analysis was undertaken. Data extracted included information about the study design, characteristics and number of participants, drug and dose regimen, permitted concomitant therapies, follow-up period, change in skin disease outcomes, adverse effects and withdrawals.
Assessment of risk of bias in included studies
During data extraction, we assessed the risk of bias in each trial included in the review using the Cochrane Collaboration's risk of bias tool.[9] This requires reviewers to evaluate the risk of five types of bias and to judge each of these as either “low,” “high,” or “unclear” (usually when there is insufficient information).[10]
Data synthesis
We described the characteristics and results of trials in table format [Table - 1] and also considered whether statistical synthesis of the findings by meta-analysis was appropriate.
Measures of treatment effect
We planned to use continuous data to calculate the mean difference between change in PASI scores in the statin and placebo groups with 95% confidence intervals using a fixed-effect model, unless significant statistical heterogeneity was found (see below). Comparing the mean PASI scores as a way of measuring treatment effect is the most common comparative tool used in psoriasis trials which use this indicator.[11],[12]
Assessment of heterogeneity
We intended to deal with statistical heterogeneity with the use of the I2 statistic provided; there were a sufficient number of eligible studies to make the interpretation of the I2 statistic reliable.[13]
Results
Results of the search
The searches yielded seven relevant studies [Figure - 1].
Included studies
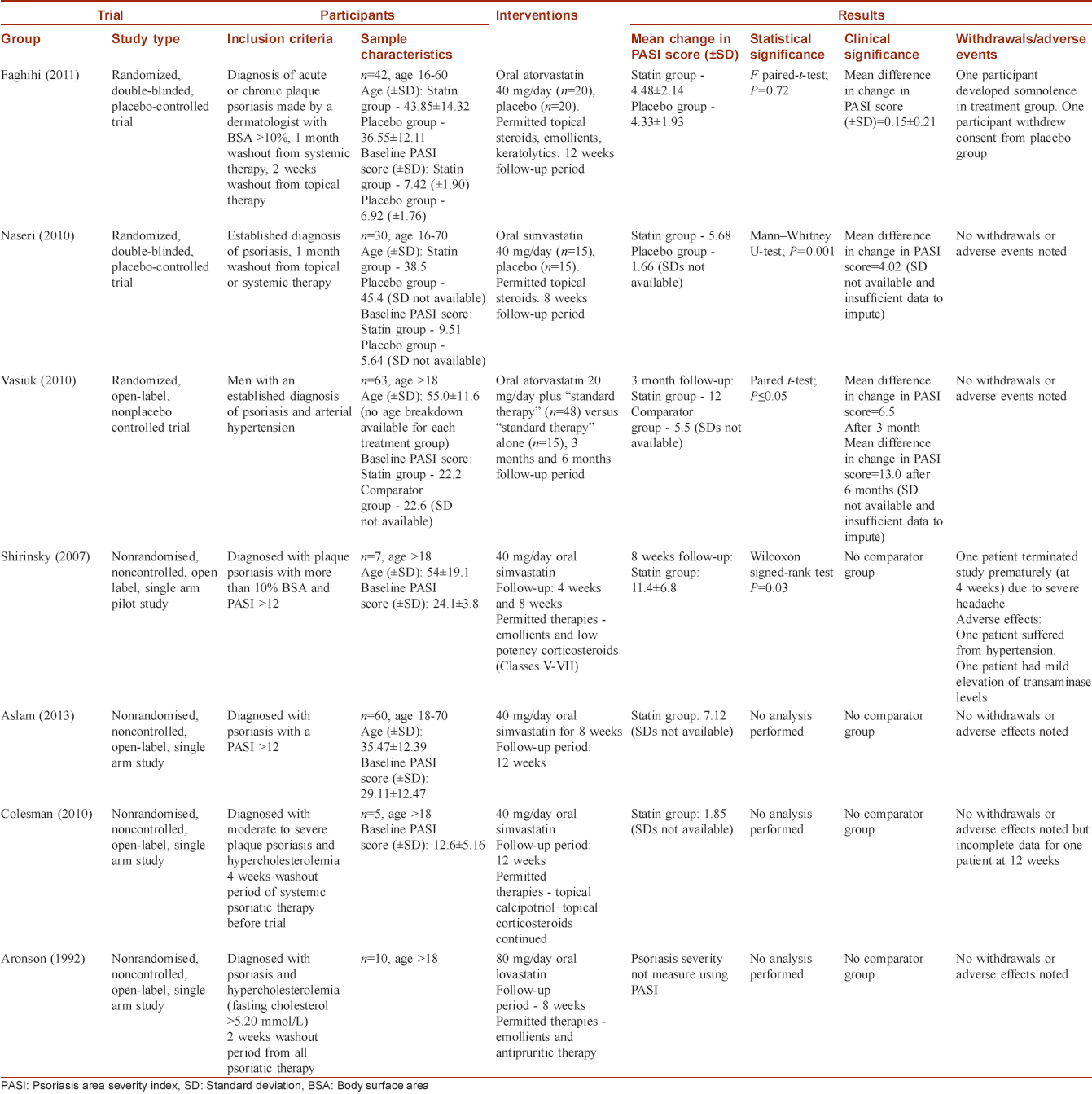
Three studies fulfilled the inclusion criteria [Table - 1]. Faghihi et al. and Naseri et al. both studied the effect of statin use on psoriasis severity using a prospective, single center, randomized, double-blinded, placebo-controlled study design and measured psoriasis severity using PASI.[14],[15] Vasiuk et al. used a prospective, single-center, randomized, open-label, nonplacebo controlled trial with parallel groups to study the effect of oral atorvastatin on psoriasis severity as measured by change in PASI score.[16]
Excluded studies
Shirinsky and Shirinsky, Aslam et al., Colsman and Sticherling, Aronson and Friedman all used a non-randomized, single-arm, open-label, study design, and were thus excluded because there was no comparator group [Table - 1].[17],[18],[19],[20] All of these studies yielded an improvement in psoriasis severity but did not provide raw data and only performed statistical analysis to show what the authors deemed a statistically significant improvement.
Risk of bias in included studies
Selection bias
Faghihi et al. adequately described their method of randomization (permuted block randomization table) but did not describe whether they performed allocation concealment. Naseri et al. and Vasiuk et al. did not comment on randomization method or whether allocation concealment was performed.
Blinding
Naseri et al. did state that the assessors of outcome were blinded to treatment identifications before the initiation of the treatment and at the end of the treatment period. Faghihi et al. did not describe any method of outcome assessment blinding, whereas Vasiuk et al. undertook an open-label study.
Incomplete outcome data
Faghihi et al. had a low withdrawal rate (2/40 participants) but did not use intention to treat analysis. This is unlikely to contribute to significant attrition bias since one participant withdrew from each treatment group and the overall withdrawal rate was low. Naseri et al. and Vasiuk et al. had no withdrawals.
Other potential sources of bias
Treatment group size was an issue.[21] The sample size was 30 for the Naseri et al. study, 40 for the Faghihi et al. study and 62 for the Vasiuk et al. study. Studies with small group sizes tend to overestimate efficacy; thus, this is a potential additional source of bias.[22] [Figure - 2] and [Figure - 3] summarise the risk of bias in included studies.
 |
| Figure 2: Risk of bias summary: Review authors' judgments about each risk of bias item for each included study |
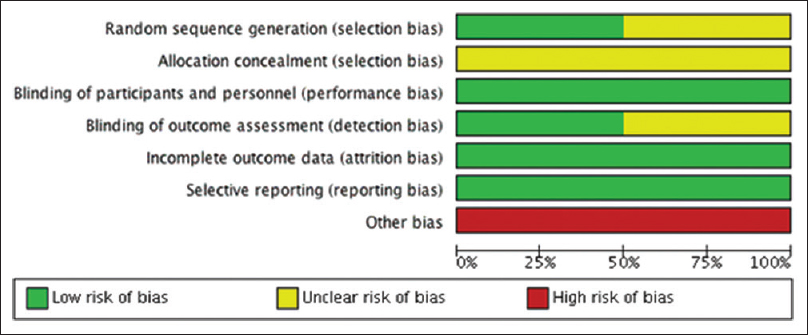 |
| Figure 3: Risk of bias' graph: Review authors' judgments about each risk of bias item presented as percentages across all included studies |
Effects of interventions
The included studies were not amenable to pooled meta-analysis because Naseri et al. and Vasiuk et al. did not assess for variation in the change in PASI score measurements.[10]
Faghihi et al. (2011) found that the mean change in PASI scores (± standard deviation) for the atorvastatin group was 4.48 ± 2.14 compared with the placebo group 4.33 ± 1.93, creating a mean difference in change in PASI score of 0.15 ± 0.21 between the two groups, which the authors stated was not statistically significant using a paired-sample t-test (P = 0.72).
Naseri et al. (2010) found that the mean change in PASI score was 5.68 in the simvastatin group and 1.66 in the placebo group creating a mean difference in change in PASI score of 4.02 between the two groups. The authors deemed this a statistically significant larger reduction in mean PASI score in the simvastatin group using a Mann–Whitney U-test statistical significance test (P = 0.001).
Vasiuk et al. (2010) found that the mean change in PASI score was 12 in the atorvastatin group and 5.5 in the placebo group after 3 months follow-up, thus creating a mean difference in change in PASI score of 6.5 between the two groups. In addition, participants were followed up at the 6-month time period yielding an even greater improvement of 13 in PASI scores.
Withdrawals and adverse effects
There were two withdrawals from the Faghihi et al. study. One participant developed somnolence in the atorvastatin group and had to discontinue the trial while another from the placebo group withdrew consent. There were no additional adverse effects noted in either treatment group. There were no withdrawals or adverse effects noted in the Naseri et al. and Vasiuk et al. studies.
Discussion
Summary of main results
This systematic review found some relevant studies; we included three and excluded another five. Naseri et al. found a significant reduction in psoriasis severity in the statin group compared with placebo. Vasiuk et al. also found a statistically significant improvement when comparing statin with standard therapy alone. Faghihi et al. found no significant difference between the groups.
The difference in results between the studies is likely to be related to differences in methodological approach. Firstly, the Faghihi et al. study required established psoriasis diagnosis by a dermatologist but did not give a description of the types of psoriasis included or excluded. In contrast, neither Naseri et al. nor Vasiuk et al. explicitly state how the diagnosis of psoriasis was verified. Furthermore, Naseri et al. excluded erythrodermic and pustular psoriasis. Secondly, the baseline psoriasis severity varied for the included participants between the populations studied, as well as between the respective treatment arms within each study. Participants had different timescales of washout periods from topical therapies and the studies varied in permitted therapies that were allowed during the study period. These factors could contribute to differences in disease severity at the onset of the treatment period, which are likely to have a significant impact on the reliability with which one can interpret results for treatment effect in the respective studies.
It is likely that there is a significant demographic difference between the study populations. Naseri et al. (2010) and Faghihi et al. (2011) both studied men and women at different centers in Iran. However, Vasiuk et al. (2010) used a very homogenous study sample (men with arterial hypertension). Some studies suggest baseline psoriasis severity tends to be worse in men as compared to women, influencing the comparability of the results. It is also feasible that there is a gender difference in response to statin therapy.[23] It is not known whether other co-morbidities, especially cardiovascular risk factors, influence statin response.
The included studies used different statins - Faghihi et al. and Vasiuk et al. used atorvastatin, whereas Naseri et al. used simvastatin. Little is known about dose equivalence in terms of the anti-inflammatory action of statins and it is possible that certain statins may be more effective than others at improving psoriasis severity.[24] Faghihi et al. reassessed participants' PASI score after a period of 12 weeks, Naseri et al. reassessed the score after 8 weeks, whereas Vasiuk et al. reassessed response after 3 months and 6 months. This may account for some of the differences in the outcome, since the time scale of potential anti-inflammatory benefit is not known. Vasiuk et al. found a significantly greater reduction in psoriasis severity in the statin group which appeared to continue up to a 6-month period. This may suggest that the anti-inflammatory action of statins may endure beyond 3 months and thus has implications for interpreting the negative findings of the Faghihi et al. study which had a follow-up period of only 12 weeks.
Furthermore, the included studies differed in their statistical approach using different methods to assess statistical significance. None of the included studies stated whether measurements followed an approximate normal distribution. In addition, Faghihi et al. controlled for baseline characteristics unlike the other two included studies.
In terms of adverse effects, the statins were generally well tolerated with only one patient dropping out from the statin arm of the Faghihi et al. study (due to somnolence which is not a known side effect of atorvastatin).[25] There were no withdrawals from the other two included studies. These results are in accordance with the good tolerability of statins when used for their lipid-lowering effect.[26]
Quality of the evidence
The “risk of bias” assessment showed that all the included studies were at high risk due to small sample size and unclear risk of selection bias via potential lack of allocation concealment. Faghihi et al. did not describe a method of outcome assessment blinding and thus the risk of detection bias was unclear, whereas the other two studies did not specify a method of random sequence generation.[21] The possibility of publication bias from unpublished negative results cannot be excluded. This may have potentially large effects on any overall assessment given the paucity of any positive results.
Excluded studies
All of the excluded studies found an improvement in psoriasis severity in their respective statin groups and the statins were well tolerated which is in line with the findings of the included studies.
Shirinsky et al. used an open-label, single arm study design with seven participants, putting it at high risk of selection, performance and detection bias. Furthermore, the lack of a comparator made it difficult to ascertain whether the improvement in psoriasis severity was related to commencing the statin, or as a response to the permitted therapies used in the treatment period.[17] Colesman et al., Aronsen et al. and Shirinsky et al. used very small study samples; their results need to be interpreted with caution since they are likely to be vulnerable to the random play of chance.[17],[19],[20]
Conclusions
Limitations
Included studies were of limited sample size and quality. They were not amenable to pooled analysis.
Implications for practice
There is insufficient evidence that the use of oral statins as an adjunctive therapy, even though well tolerated, can reduce the severity of psoriasis. There is only one placebo-controlled, randomized control trial to date that has shown that they may be beneficial in reducing the severity of psoriasis.
Implications for research
This review highlights the paucity of high quality, randomized, double-blinded placebo-controlled trials investigating the effects of statins on psoriasis severity using clinically objective measures. Since these are well-tolerated drugs with promising non-randomised single arm trial results, it seems possible that larger evidence-based trials can be conducted. Future studies need to ensure that enrolled participants have a standardized diagnosis of psoriasis, preferably by a dermatologist, and need to be larger in size to ensure adequate statistical power. It would also be advisable for future studies to control for baseline characteristics since there is some evidence that gender differences may affect baseline psoriasis severity and thus, potentially affect disease response. Furthermore, it is feasible that a statin-induced disease response may extend beyond a 1–2 month time period and longer follow-up periods may detect delayed response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Christophers E. Psoriasis – Epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314-20.
[Google Scholar]
|
| 2. |
Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 1996;14:485-96.
[Google Scholar]
|
| 3. |
Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. J Am Acad Dermatol 2007;57:347-54.
[Google Scholar]
|
| 4. |
Samarasekera E, Sawyer L, Parnham J, Smith CH; Guideline Development Group. Assessment and management of psoriasis: Summary of NICE guidance. BMJ. 2012 Oct 24;345:e6712.
[Google Scholar]
|
| 5. |
Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): The correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol 2014;28:333-7.
[Google Scholar]
|
| 6. |
Istvan ES. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Am Heart J 2002;144 6 Suppl: S27-32.
[Google Scholar]
|
| 7. |
Ginter E, Simko V. Statins: The drugs for the 21st century? Bratisl Lek Listy 2009;110:664-8.
[Google Scholar]
|
| 8. |
Corsonello A, Garasto S, Abbatecola AM, Rose G, Passarino G, Mazzei B, et al. Targeting inflammation to slow or delay functional decline: Where are we? Biogerontology 2010;11:603-14.
[Google Scholar]
|
| 9. |
Hopp L. Risk of bias reporting in Cochrane systematic reviews. Int J Nurs Pract 2015;21:683-6.
[Google Scholar]
|
| 10. |
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
[Google Scholar]
|
| 11. |
Micali G, Wilsmann-Theis D, Mallbris L, Gallo G, Marino V, Brault Y, et al. Etanercept reduces symptoms and severity of psoriasis after cessation of cyclosporine therapy: Results of the SCORE study. Acta Derm Venereol 2015;95:57-61.
[Google Scholar]
|
| 12. |
Lajevardi V, Hallaji Z, Daklan S, Abedini R, Goodarzi A, Abdolreza M. The efficacy of methotrexate plus pioglitazone vs. methotrexate alone in the management of patients with plaque-type psoriasis: A single-blinded randomized controlled trial. Int J Dermatol 2015;54:95-101.
[Google Scholar]
|
| 13. |
L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med 1987;107:224-33.
[Google Scholar]
|
| 14. |
Faghihi T, Radfar M, Mehrabian Z, Ehsani AH, Rezaei Hemami M. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy 2011;31:1045-50.
[Google Scholar]
|
| 15. |
Naseri M, Hadipour A, Sepaskhah M, Namazi MR. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis: A double-blind, randomized, placebo-controlled study. Niger J Med 2010;19:58-61.
[Google Scholar]
|
| 16. |
Vasiuk IU, Perlamutrov IU, Shkol'nik MN, Shkol'nik EL. Possibilities of atorvastatin in complex management of extensive psoriasis in patients with arterial hypertension. Kardiologiia 2010;50:37-46.
[Google Scholar]
|
| 17. |
Shirinsky IV, Shirinsky VS. Efficacy of simvastatin in plaque psoriasis: A pilot study. J Am Acad Dermatol 2007;57:529-31.
[Google Scholar]
|
| 18. |
Aslam S, Khurshid K, Asad F, Rani Z, Pal SS. Efficacy and safety of simvastatin in chronic plaque psoriasis. J Pak Assoc Dermatol 2013;23:310-4.
[Google Scholar]
|
| 19. |
Colsman A, Sticherling M. Simvastatin in psoriasis: Ambiguous effects. Acta Derm Venereol 2010;90:411.
[Google Scholar]
|
| 20. |
Aronson PJ, Friedman DB. Pharmacologic doses of lovastatin do not predictably affect the course of psoriasis. Arch Dermatol 1992;128:124.
[Google Scholar]
|
| 21. |
Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev 2002;24:39-53.
[Google Scholar]
|
| 22. |
Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9.
[Google Scholar]
|
| 23. |
Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: Rheumatoid arthritis, inflammatory bowel disease and psoriasis: An observational study. BMC Med 2012;10:82.
[Google Scholar]
|
| 24. |
Kim TG, Byamba D, Wu WH, Lee MG. Statins inhibit chemotactic interaction between CCL20 and CCR6 in vitro: Possible relevance to psoriasis treatment. Exp Dermatol 2011;20:855-7.
[Google Scholar]
|
| 25. |
Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 2013;6:390-9.
[Google Scholar]
|
| 26. |
Šimic I, Reiner Ž. Adverse effects of statins – Myths and reality. Curr Pharm Des 2015;21:1220-6.
[Google Scholar]
|
Fulltext Views
6,196
PDF downloads
2,184





