Translate this page into:
The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia
2 Intermedica Clinic for Hair Diseases, Department of Dermatology, Boxmeer; Maasstadziekenhuis, Rotterdam, Dermicis, Alkmaar, Erasmus MC-Sophia, Rotterdam, The Netherlands
3 Department of Dermatology, Faculty of Medicine, International University of Catalonia, Sant Cugat, Barcelona, Spain
4 Department of Dermatology, Private practice, Bellinzona, Switzerland
5 Dermatology Department of Specialized, Experimental and Diagnostic Medicine, University of Bologna, Bologna, Italy
Correspondence Address:
Emiel H Verdonschot
Intermedica Clinic, Department of Dermatology, Dr. Peelenstraat 8, 5831EG Boxmeer
The Netherlands
| How to cite this article: Boersma IH, Oranje AP, Grimalt R, Iorizzo M, Piraccini BM, Verdonschot EH. The effectiveness of finasteride and dutasteride used for 3 years in women with androgenetic alopecia . Indian J Dermatol Venereol Leprol 2014;80:521-525 |
Abstract
Background: The effectiveness of finasteride and dutasteride in women with androgenetic alopecia has been the subject of debate. Aim: To evaluate the effectiveness of finasteride and dutasteride on hair loss in women with androgenetic alopecia over a period of 3 years. Methods: From a database containing systematically retrieved data on 3500 women treated for androgenetic alopecia between 2002 and 2012 with finasteride 1.25 mg or dutasteride 0.15 mg, a random sample stratified for age and type of medication was taken to yield 30 women in two age categories: below and above 50 years, and for both medications. Hair thickness of the three thinnest hairs was measured from standardized microscopic images at three sites of the scalp at the start of the treatment and after 3 years of continuous medication intake. The macroscopic images were evaluated independently by three European dermatologists/hair experts. The diagnostic task was to identify the image displaying superior density of the hair. Results: Both age categories showed a statistically significant increase in hair thickness from baseline over the 3-year period for finasteride and dutasteride (signed rank test, P = 0.02). Hair thickness increase was observed in 49 (81.7%) women in the finasteride group and in 50 (83.3%) women in the dutasteride group. On average, the number of post-treatment images rated as displaying superior density was 124 (68.9%) in the finasteride group, and 118 (65.6%) in the dutasteride group. Dutasteride performed statistically significantly better than finasteride in the age category below 50 years at the central and vertex sites of the scalp. Conclusions: Finasteride 1.25 mg and dutasteride 0.15 mg given daily for 3 years effectively increased hair thickness and arrested further deterioration in women with androgenetic alopecia.INTRODUCTION
Androgenetic alopecia or female pattern hair loss is a very common condition in women, generally presenting from the age of 40 years on. It affects 40% of the women over 70 years of age, [1] and may already be noted in adolescent females. [2] To most of the affected women, the baldness is perceived as a major cosmetic inconvenience and a significant psychological problem. [3]
The effectiveness of finasteride in arresting hair loss due to androgenetic alopecia in women has been the subject of three prospective randomized controlled trials (RCTs), all of which concluded that the drug was ineffective. [4],[5],[6] A recent Cochrane systematic review on the treatment of androgenetic alopecia in women concluded that these three RCTs were associated with a high risk of bias, and therefore could not be used to support recommendations in clinical guidelines on this subject. [7] Moreover, the effect measure chosen, i.e. increase of hair density, and the short duration of the studies up to a maximum of 18 months further diminish their value for use in supporting clinical decision making. Clinical studies with designs other than a randomized controlled trial were not considered in this Cochrane review, yet these publications contain support for the effective and safe use of finasteride and dutasteride. [8],[9],[10],[11],[12]
The absence of solid evidence for the effectiveness of finasteride and dutasteride leaves clinicians in doubt. Many publications recommend that new well-designed randomized trials should be conducted to clarify the issue but no such studies have appeared after the publication of the aforementioned Cochrane review. We report on the effectiveness of finasteride and dutasteride prescribed to women in a dermatology clinic specialized in hair disorders.
METHODS
Patient population
Between 2002 and 2012, approximately 3500 women diagnosed with androgenetic alopecia were prescribed finasteride 1.25 mg or dutasteride 0.15 mg daily after written informed consent. The choice of doses was derived from an internally conducted dose finding study. The age range was 16-84 years. A yearly general clinical examination and a blood sample analysis were done. In premenopausal women, the prescription of finasteride or dutasteride was combined with contraceptive measures. Routine data collected at all patient visits comprised standardized global photography of the scalp and direct microscopic images at the center of the frontal hairline, at the center of the scalp at the midline, and at the vertex (Mirror system, Canfield Imaging Systems, Fairfield, NJ, USA). The imaging system allowed for the production of reproducible images of the scalp at each visit. A software tool enabled the measurement of the hair thickness from the microscopic images. From the standardized microscopic images at three sites of the scalp the hair thickness of the three thinnest hairs was measured at the start of the treatment and after 3 years of continuous medication intake.
The retrospective design of this study did not allow a priori consent by an ethics committee. Information related to the identity of the patients was disclosed to all participating researchers.
Sampling
Criteria for inclusion in this study were:
- A clinical diagnosis of Ludwig grade 1 or grade 2 androgenetic alopecia confirmed by the presence of thin hair on the microscopic picture
- Continuous treatment for at least 3 years, assessed by the regular filling of the prescription
- A stratified random sample of 60 patients taking finasteride and 60 patients taking dutasteride was taken for analysis. Both groups contained 30 patients aged below 50 years and 30 patients aged 50 years or above.
Hair thickness
Thickness measurements of the three thinnest hairs at each site were performed by two trained analysts. Intra-observer reliability was assessed by duplicate measurement of 20 cases. The increase in hair thickness was obtained by subtracting the pretreatment hair thickness from that measured after 3 years. Treatment was considered effective if the hair thickness increase was equal to, or greater than zero.
Scalp coverage and hair structure
The standardized global macroscopic images of the center of the scalp and the vertex were evaluated independently by three European hair experts. Twenty duplicate cases were included to establish the intra-observer reliability. The pre- and post-treatment images were displayed next to each other. Based on randomly assigned sequence numbers, the pre-treatment images were mounted to the left or right, with the post-treatment image to the other side. If a pre-treatment image was identified as displaying superior scalp coverage and structure compared to the post-treatment image, it was scored as -1. Pre- and post-treatment images assessed as equal in terms of scalp coverage and structure were scored as 0, whereas a score of +1 was assigned if a post-treatment image was judged to display superior scalp coverage and structure. Scores for the center of the scalp and the vertex region were averaged to obtain one patient score. Treatment was considered effective for patient scores equal to, or larger than zero.
Statistical analysis
Differences between original measurements and duplicate measurement of hair thickness were tested using a paired Student t-test and Pearson correlation coefficients. Signed rank tests at α = 0.05 were conducted to test whether the hair thickness increase was different from zero, and to test differences between hair thickness increase by finasteride and dutasteride. Intra-observer reliability of the scalp coverage scores by the hair experts was expressed as Cohen′s kappa statistic between the scores for the original images and the duplicate images. Signed rank tests at α = 0.05 were conducted to test whether the patient scores from the hair experts were statistically significantly different from zero.
RESULTS
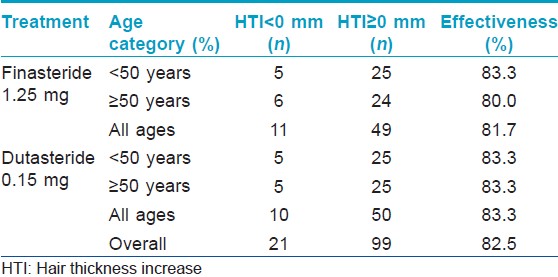
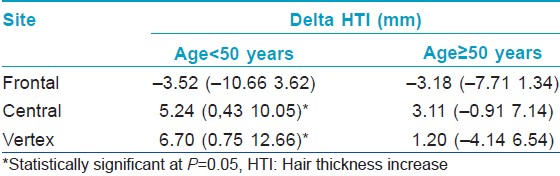
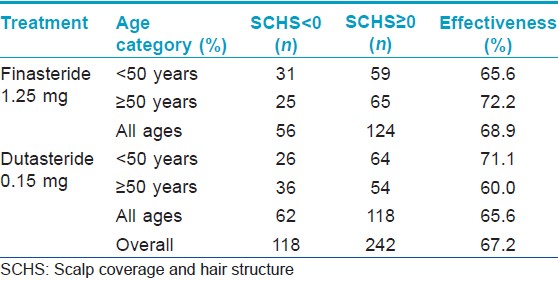
[Figure - 1] and [Figure - 2] depict the results of hair thickness increase after 3 years of finasteride 1.25 mg and dutasteride 0.15 mg respectively. There was no statistically significant difference between original and duplicate measurements of hair thickness by the two analysts (paired student t-test; P = 0.86) and linear correlation was high (Pearson correlation coefficients are 0.89 and 0.96, respectively) indicating excellent intra-observer reliability. A total of 49 (81.7%) women in the finasteride group and 50 (83.3%) in the dutasteride group had hair thickness increase equal to or greater than 0. These numbers were independent of age [Table - 1]. All hair thickness increases were statistically significantly different from 0 in both age categories and at all sites of the scalp (Signed rank test, P < 0.05), except for the increase in the age category above 50 years by dutasteride at the frontal site. The differences in hair thickness increase between dutasteride and finasteride were significantly higher with higher means for dutasteride users in the age group under 50 years at the center of the scalp and the vertex (Signed rank test, P < 0.05), indicating a superior effectiveness of dutasteride at these two sites [Table - 2].
Cohen′s kappa statistics for subjective scalp coverage and structure assessments were 0.50, 0.70 and 0.70 for the three hair experts indicating fair intra-observer reliability. The number of image pairs associated with improved hair density, subjectively assessed by three hair experts, was 124 (68.9%) in the finasteride group and 118 (65.6%) in the dutasteride group. The effectiveness was highest in the age category above 50 years for finasteride, whereas dutasteride reached its highest effectiveness in the age category below 50 years [Table - 3].
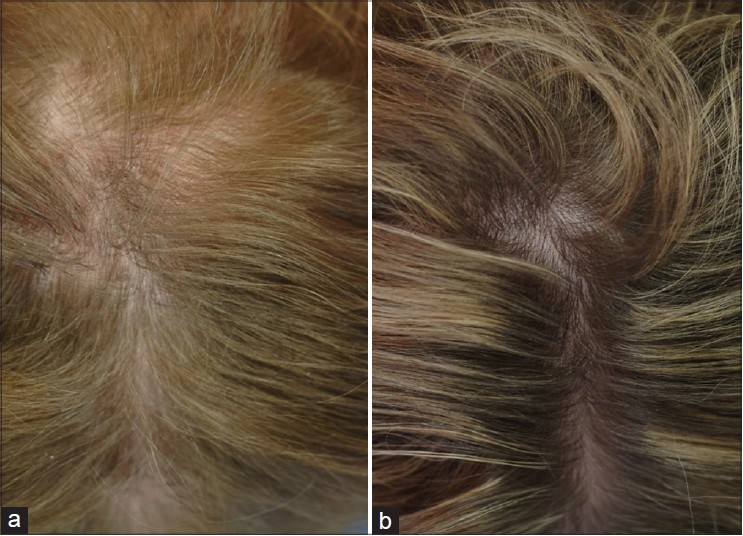 |
| Figure 1: Central view of the scalp of a 57-year-old woman before (a) and after (b) the use of finasteride 1.25 mg daily for 3 years |
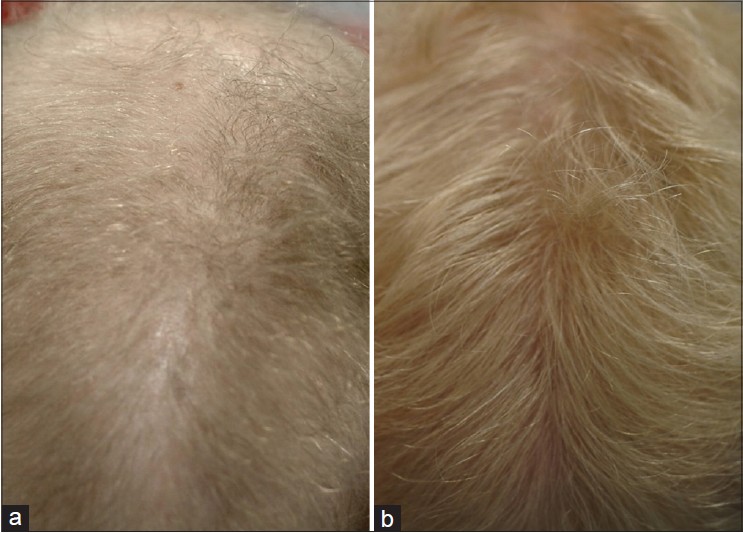 |
| Figure 2: Central view of the scalp of a 22-year-old woman before (a) and after (b) the use of dutasteride 0.15 mg daily for 3 years |
DISCUSSION
The effectiveness of finasteride in the prevention of hair loss in men has been well established [13] and its prescription to balding male persons has become an accepted treatment option. Finasteride is a type II 5-alpha-reductase inhibitor which decreases dihydroxytestosterone (DHT) by about 65% in serum, prostate and scalp. Dutasteride inhibits both type I and type II 5-alpha-reductase resulting in a decrease of the serum dihydroxytestosterone (DHT) level of about 90%, and has a well-established effectiveness in the treatment of androgenetic alopecia in males. [14],[15] The prescription of finasteride and dutasteride to women with female pattern hair loss proceeds from the premise that hair loss in women results from the same process as in male subjects. This study demonstrates that, like in men, the reduction of hair thickness and resulting hair loss in women with androgenetic alopecia could be stopped or reversed by 5-alpha-reductase inhibitors.
This study is one of very few in which hair thickness was used as an effect measure to judge the clinical effectiveness of finasteride or dutasteride. We did not select hair density as an effect measure, as no scientific reports exist that the number of hair per unit area should increase due to the use of finasteride or dutasteride. However, there is considerable evidence that the size of hair follicles, hair thickness and hair growth will increase following finasteride and dutasteride treatment for more than 1 year, which justified the choice for selection of hair thickness to measure the effect of the treatment. Moreover, the increase of thickness of the thinnest hair observed in women in this study supports the theory of the androgen-dependent nature of hair loss, which was questioned by Olsen. [16]
Androgenetic alopecia in women is clinically characterized by slowly progressive hair thinning, occurring over years. The goal of medical treatment is to arrest progression of the disease and to improve hair thickness and density. This goal can be reached only with prolonged treatment since regrowth of thicker hair requires years rather than months. Moreover, a treatment should also be regarded as successful if further deterioration of the hair can be prevented, not only if improvement of hair thickness and length is obtained. In our study, both finasteride and dutasteride were able to arrest progression of androgenetic alopecia and to improve the condition in 68.9% and 65.6% of the cases, respectively, when determined subjectively after 3 years of treatment. The effectiveness expressed by objectively established hair thickness increase was 81.7% and 83.3%, respectively. This effect size is well in line with those from studies on the effectiveness of finasteride and dutasteride in males. [13],[17]
Dutasteride 0.15 mg appears significantly more effective than finasteride 1.25 mg in women under 50 years of age when measured at the center of the midline and the vertex. This finding indicates that dutasteride should preferably be prescribed to younger women. To preclude teratogenic effects, we recommend that both finasteride and dutasteride be prescribed only to women who cannot or do not want to become pregnant any more. The latter group should take adequate contraceptive measures and the prescribing doctor should verify that patients are compliant with these measures. The absence of a significant difference in effectiveness between finasteride and dutasteride at the frontal line may be explained by the preservation of the frontal hairline which is typical for women with female pattern hair loss. Apparently, the frontal hairline consists predominantly of hairs which are not sensitive to dihydroxytestosterone (DHT).
Compared to controlled clinical trials, retrospective studies have drawbacks with the absence of a control group receiving placebo treatment being a major one. The lack of double blind data collection is a second source of bias. An additional potential source of bias was introduced by the criterion that only women who used finasteride or dutasteride for 3 years continuously were eligible for inclusion, thereby excluding women who interrupted this medication within 3 years because of perceived or ascertained ineffectiveness, side effects, financial reasons, planned pregnancy, or preference for other treatment options. However, the data from all patients in the clinic were collected systematically and routinely, as in a prospective study. Despite the disadvantages of a retrospective study, our results may contribute to the debate on the effectiveness of finasteride and dutasteride in women with androgenetic alopecia when prescribed under field circumstances, and to support the decision to conduct a new well-designed randomized controlled trial.
| 1. |
Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol 2001;144:297-304.
[Google Scholar]
|
| 2. |
Price VH. Androgenetic alopecia in adolescents. Cutis 2003;71:115-21.
[Google Scholar]
|
| 3. |
Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjects. J Am Acad Dermatol 1993;29:568-75.
[Google Scholar]
|
| 4. |
Carmina E, Lobo RA. Treatment of hyperandrogenic alopecia in women. Fertil Steril 2003;79:91-5.
[Google Scholar]
|
| 5. |
Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfeld W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol 2000;43:768-76.
[Google Scholar]
|
| 6. |
Whiting DA, Waldstreicher J, Sanchez M, Kaufman KD. Measuring reversal of hair miniaturization in androgenetic alopecia by follicular counts in horizontal sections of serial scalp biopsies: Results of finasteride 1 mg treatment of men and postmenopausal women. J Investig Dermatol Symp Proc 1999;4:282-4.
[Google Scholar]
|
| 7. |
Zuuren EJ van, Fedorowicz Z, Carter B, Andriolo RB, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev 2012;5:CD007628.
[Google Scholar]
|
| 8. |
Trüeb RM, Swiss Trichology Study Group. Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatology 2004;209:202-7.
[Google Scholar]
|
| 9. |
Yeon JH, Jung JY, Choi JW, Kim BJ, Youn SW, Park KC, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol 2011;25:211-4.
[Google Scholar]
|
| 10. |
Stout SM, Stumpf JL. Finasteride treatment of hair loss in women. Ann Pharmacother 2010;44:1090-7.
[Google Scholar]
|
| 11. |
Iorizzo M, Vincenzi C, Voudouris S, Piraccini BM, Tosti A. Finasteride treatment of female pattern hair loss. Arch Dermatol 2006;142:298-302.
[Google Scholar]
|
| 12. |
Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol 2005;4:637-40.
[Google Scholar]
|
| 13. |
Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, Trakatelli M, et al. S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 2011;164:5-15.
[Google Scholar]
|
| 14. |
Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: A randomized, double-blind, placebo-controlled phase III study. J Am Acad Dermatol 2010;63:252-8.
[Google Scholar]
|
| 15. |
Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: Results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol 2006;55:1014-23.
[Google Scholar]
|
| 16. |
Olsen EA. Female pattern hair loss. J Am Acad Dermatol 2001;45(Suppl):S70-80.
[Google Scholar]
|
| 17. |
Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol 1998;39:578-89.
[Google Scholar]
|
Fulltext Views
38,612
PDF downloads
2,824





