Translate this page into:
The efficacy of topical adipose mesenchymal stem cell-conditioned medium versus framycetin gauze dressing in chronic plantar ulcer of leprosy: A randomized controlled trial
Corresponding author: Dr. Medhi Denisa Alinda, Jl. Mayjen Prof. Dr. Moestopo 47, Surabaya 60132, Indonesia. medhidenisaalinda@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Alinda MD, Christopher PM, Listiawan MY, Endaryanto A, Suroto H, Rantam FA, et al. The efficacy of topical adipose mesenchymal stem cell-conditioned medium versus framycetin gauze dressing in chronic plantar ulcer of leprosy: A randomized controlled trial. Indian J Dermatol Venereol Leprol 2023;89:656-64
Abstract
Background
Wound healing shows a unique interaction of several cells, growth factors and cytokines. The healing of chronic plantar ulcer of leprosy is influenced by various factors, one of which is the concentration of growth factors and cytokines related to the pathogenesis of impaired wound healing. Growth factors and cytokines can be found in the secretome of adipose mesenchymal stem cells.
Aim
To compare the effectiveness of topical adipose mesenchymal stem cell-conditioned medium and framycetin gauze dressing only on the healing of chronic plantar ulcer of leprosy.
Methods
In this randomised controlled trial, 32 patients with chronic plantar ulcer of leprosy were recruited. After detailed clinical and initial debridement, patients were randomised to two groups to receive either topical adipose mesenchymal stem cell-conditioned medium (n = 16) or framycetin gauze dressing only (n = 16) applied every three days for up to eight weeks, following which the ulcer size, adverse reactions and complications if any were monitored weekly.
Results
Healing percentage increased each week in all groups. Statistical differences between groups (P < 0.05) were observed from week 2 onwards for ulcer mean size reduction and from week 3 onwards for ulcer mean depth reduction. There were no adverse reactions or complications.
Limitations
Off-loading on subjects were not performed.
Conclusion
Adipose mesenchymal stem cell-conditioned medium is a potential therapeutic agent in the management of chronic plantar ulcer of leprosy.
Keywords
Adipose mesenchymal stem cell-conditioned medium
chronic plantar ulcer of leprosy
leprosy
tropical disease
Plain Language Summary
Leprosy, a chronic granulomatous curable disease caused by Mycobacterium leprae infecting the mucocutaneous tissues and peripheral nerves, may lead to complications, one of which is the chronic plantar ulcer of leprosy (CPUL), one of the most common complications of leprosy due to the impairment of sensation in the foot. Recent studies have found adipose mesenchymal stem cell-conditioned medium (ADMSC-CM) to be a novel therapeutic agent with its reparative and regenerative properties. We conducted a study in a hospital in Surabaya, Indonesia, to compare it with framycetin gauze dressing (FGD) to manage chronic plantar ulcer of leprosy. Patients were distributed into two groups of 16 each. The first group was treated with topical adipose mesenchymal stem cell-conditioned medium and the second group was treated with framycetin gauze dressing; each was applied every three days for eight weeks. Ulcer size, adverse reactions and possible complications were monitored weekly. Demographics and baseline characteristics of the patients were similar at baseline. A significant difference in ulcer mean size and depth reduction were observed from weeks two and three onwards, respectively. No adverse reaction was noted in both groups. This study showed that adipose mesenchymal stem cell-conditioned medium has a better outcome in healing chronic plantar ulcer of leprosy for ulcer size and depth. Thus, it is a potential therapeutic agent in the management of such ulcers.
INTRODUCTION
An ulcer is defined as a loss of continuity of skin tissue up to the dermis or deeper (subcutaneous tissue).1 Approximately 30% of patients with leprosy have ulcers due to peripheral nerve damage.2 The trophic ulcer or chronic plantar ulcer of leprosy, refers to the dreaded, recalcitrant disability of the sensory-deficient foot, mostly in plantar area, particularly in areas with bony prominence. Involvement and dysfunction of the peripheral nerves result in skin anaesthesia, and may subsequently lead to disabilities, including ulcers.3 The chronicity of plantar ulcer of leprosy is further exacerbated through repeated inadvertent trauma or injury.4 The moment ulceration occurs, the foot becomes ‘ulcer prone’ and the vicious cycle of scar-ulcer-scar takes place.5
To help a chronic wound, heal naturally is often challenging and time-consuming due to the decreased level of cytokines and growth factors because of the protracted inflammatory condition. Infection and ischemia induced by continuous pressure on the wound from disruption of nerve functions might exacerbate the inflammatory disease. This causes obstruction of the wound-healing process and further enhancing the formation of a chronic ulcer.6
Over the last few years, mesenchymal stem/stromal cells have emerged as a promising therapeutic option. Mesenchymal stem/stromal cells are fibroblast-like cells present in various adult tissues with the property of high degree plasticity and are able to differentiate into various ecto-, endo- and mesodermal lineages.7 Mesenchymal stem/stromal cells are a heterogeneous population that was first discovered in the bone marrow-mesenchymal stem/stromal cells, subsequently obtained from various adult tissues, i.e., adipose tissues, placenta, dental pulp and umbilical cord. Studies on mesenchymal stem/stromal cells, specifically adipose mesenchymal stem cells for repair and regeneration of cells have been done in many diseases with promising results, such as osteoarthritis, diabetes mellitus, multiple sclerosis, cardiovascular disease and inflammatory bowel disease, among others.8-12 The production of growth factors and cytokines, which are essential for wound healing, is one of the mechanisms of stem cells in the tissue healing process.13
An in vivo study comparing the efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium and a mixture of topical human amniotic membrane-mesenchymal stem cell-conditioned medium + vitamin C and human amniotic membrane-mesenchymal stem cell-conditioned medium + vitamin E on chronic plantar ulcers in leprosy applied every three days for up to 8 weeks yielded an increased healing percentage each week in all groups with the highest in the human amniotic membrane-mesenchymal stem cell-conditioned medium + vitamin E group clinically and statistically (P < 0.000 and P < 0.000).14
Upon considering the facts, in this study, we investigated and compared the effect of topical gel adipose mesenchymal stem cell-conditioned medium-conditioned medium (group 1) and framycetin gauze dressing only (group 2), growth factors (transforming growth factor-β [TGF-β]) and cells (macrophages, fibroblasts and vascular) on the healing of chronic plantar ulcer of leprosy.
MATERIALS AND METHODS
Sample size calculation
A sample size of 16 per group was required to achieve a 95% difference in the ulcer size and depth at eight weeks between groups 1 and 2 for the management of chronic plantar ulcer of leprosy with 5% alpha and 80% power.14
Patient recruitment
This experimental analytical study implemented a single-blinded randomised controlled clinical trial involving 16 subjects in each of the two treatment groups. Consecutive sampling was performed, i.e., we enrolled any patient with chronic plantar ulcer of leprosy who met the inclusion criteria for admission at the Dermatology and Venereology outpatient clinic of Dr. Soetomo Teaching Hospital. The inclusion criteria were leprosy patients with chronic plantar ulcer of leprosy of more than six weeks, an ulcer depth of <0.5 cm, and a maximum injury area of 9 cm2 and who was not on systemic corticosteroids for at least previous two weeks. The subjects had no history of diabetes mellitus, haemophilia, blood clotting physiological disorder, antiplatelet use or hypersensitivity to transparent film dressings or adhesive plasters.
This clinical study was reviewed and approved by the ethical committee of Dr. Soetomo Teaching Hospital Surabaya (Trial Registration Number: 0052/LOE/302.4.2/VII/2020). All participants provided the written informed consent after a detailed discussion about the treatment plan, expected outcome, benefits and risk of treatment.
Pre-treatment evaluation
A detailed history and clinical evaluation were undertaken during the first visit. Ulcer size and depth were determined to assess the baseline data. Pre-treatment clinical photographs of the affected sites were taken. Prior to the application of the designated intervention, surgical debridement was performed in both groups to remove callus and necrotic tissue and return all ulcers to the same healing phase, i.e., the coagulation and inflammatory phase.
Randomisation of treatment arms
All eligible patients with normal baseline investigations were randomly allocated to two groups, group 1 and 2 (1:1) [Figure 1]. The investigators were unmasked about the drug given. Participants were blinded to the drug given. The randomisation was done by another consultant in the department who was not associated with the study. The drug was applied every three days until the ulcer closed or for a maximum of eight weeks. Off-loading was not performed in any subjects. Subjects were advised to reduce standing and walking activities.
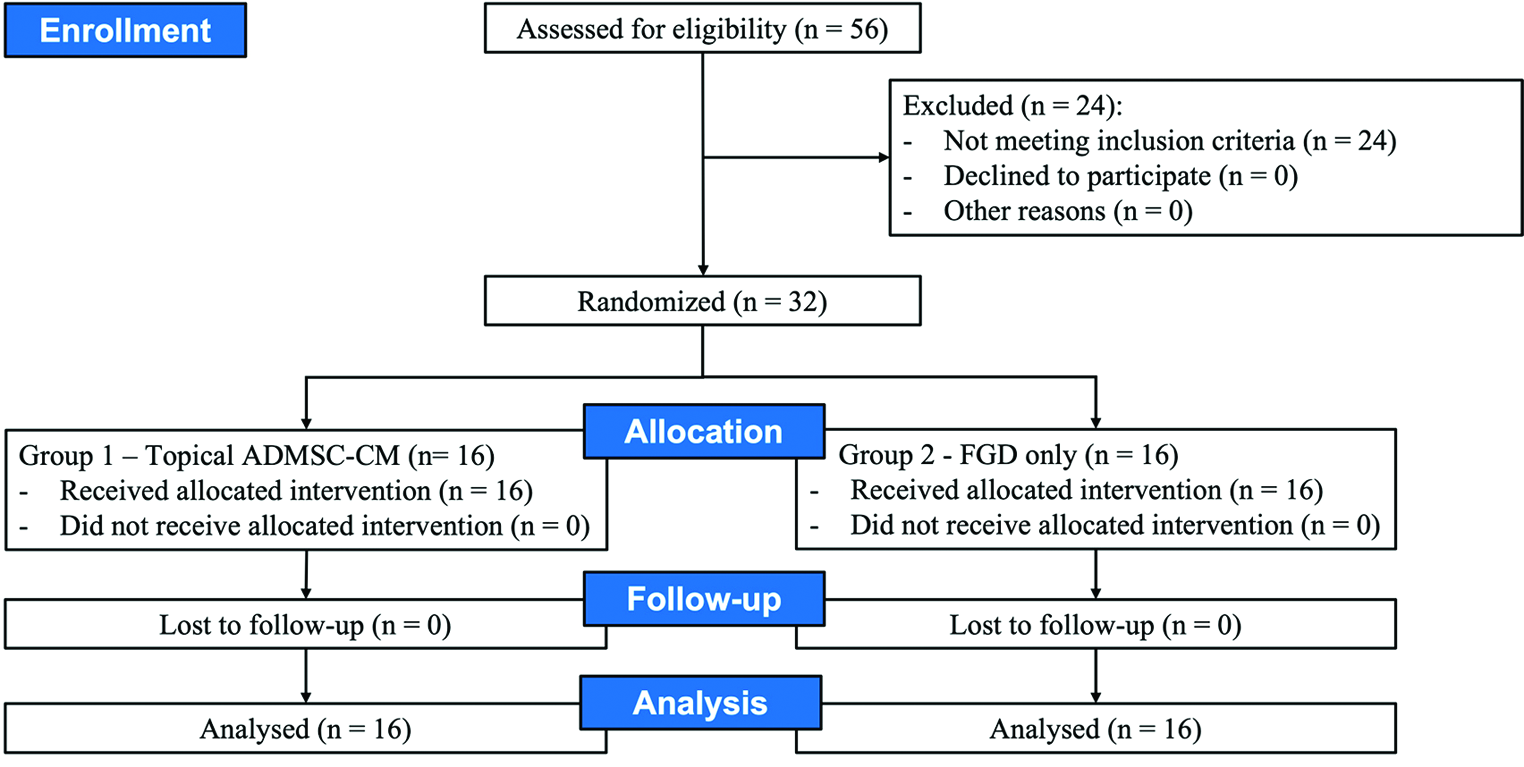
- Study flow chart showing randomisation, treatment assignment, follow-up and analysis
Laboratory testing
Extraction and preparation of adipocyte-derived mesenchymal stem cells-conditioned medium
The extraction and preparation of adipose mesenchymal stem cell-conditioned medium followed the standard procedure of (1) fat tissue sample preparation, (2) mesenchymal stem/stromal cells isolation from fat tissue process, (3) propagation of mesenchymal stem/stromal cells from isolated fat tissue, (4) characterisation of mesenchymal stem/stromal cells from fat tissue and (5) harvesting metabolites.
Histopathological examination
Histopathological examination was used to evaluate macrophage, fibroblast and vascularity. Biopsy for histopathological examination was done from the ulcer area and tissue obtained was fixed in 10% formalin and stained with haematoxylin-eosin staining.
Immunohistochemistry
Immunohistochemistry was utilised to evaluate TGF-β using expression analysis.
Follow-up
The patients were monitored weekly for the extent and ulcer depth measurement and evaluation of side effects or complications. The evaluation was done by a single observer. Clinical evaluations were repeated every week to monitor the clinical response and adverse effects. Post-treatment photographs were taken for evaluation of visual outcome and comparison in each patient.
Outcomes
The outcomes examined were the core outcome domains for ulcer healing.
Primary outcome
The primary outcome was investigator-reported treatment success of the target ulcer after eight weeks of treatment. This was measured clinically, with treatment success defined as ‘fully healed’ or ‘improved’ compared with before treatment. Investigators used digital images of the target ulcer taken before treatment to help their assessment.
Secondary outcome
Secondary outcomes were (1) immunohistochemistry and microscopic analysis of TGF-β, macrophages, fibroblasts and vascularity at the start- and the end of the eighth week of the intervention, and (2) adverse reactions during the treatment phase.
Statistical analysis
Statistical analyses were performed using SPSS (Statistical Packages for Social Sciences) version 20 (USA). Data were presented as number (%) or mean ± SD/median (min-max), as appropriate. Statistical analysis was performed with the nonparametric Mann-Whitney U test to determine significant differences between samples before and after treatment within the same group and between two groups. A P value of < 0.05 were considered statistically significant.
RESULTS
A total of 32 patients (19 male and 13 female), from August to November 2020, were included in the study. The mean age of patients was 45.47 ± 6.10 years, and the mean ± SD ulcer size and depth at baseline were 1.67 ± 1.52 cm2 and 0.71 ± 0.95 cm, respectively [Table 1]. There were no dropouts in the study. All the patients who were included completed the study protocol.
| Variable | Group 1 (n = 16) | Group 2 (n = 16) | P |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 10 (62.5) | 9 (56.2) | 1.000 |
| Female, n (%) | 6 (37.5) | 7 (43.8) | |
| Age | |||
| Min-max | 38-52 | 32-54 | 0.799 |
| Mean ± SD | 45.75 ± 4.97 | 45.19 ± 7.22 | |
| Occupation | |||
| Requires long-standing, n (%) | 7 (43.8) | 7 (43.8) | 1.000 |
| Does not require long-standing, n (%) | 9 (56.2) | 9 (56.2) | |
| Ulcer location | |||
| Fore foot, n (%) | 11 (68.8) | 10 (62.5) | 0.909 |
| Mid foot, n (%) | 2 (12.5) | 2 (12.5) | |
| Hind foot, n (%) | 3 (18.8) | 4 (25.0) | |
Response to treatment
The patients were monitored weekly for the extent and ulcer depth measurement and evaluation of side effects and complications of the drugs. The mean percentage of ulcer healing per week between groups is shown in Figures 2 and 3. The healing percentage increased each week in all the groups. At the end of the study, mean reduction in ulcer size and depth was 1.13 ± 0.65 cm2 and 0.61 ± 0.82 cm2 and 0.63 ± 0.03 cm2 and 0.62 ± 0.59 cm in group 1 and 2, respectively [Table 2]. Clinical picture before and after the application of the gel in each group is shown in Figures 4a-b and 5a-b.
| Group 1 (n = 16) | Group 2 (n = 16) | P | |
|---|---|---|---|
| The mean size reduction of the ulcer ± SD | |||
| Week I ± SD | 1.37 ± 1.20 | 1.95 ± 1.81 | 0.274 |
| Week II ± SD | 0.94 ± 1.06 | 1.97 ± 1.80 | 0.036 |
| Week III ± SD | 0.68 ± 0.88 | 2.08 ± 1.94 | 0.008 |
| Week IV ± SD | 0.66 ± 1.02 | 1.84 ± 1.92 | 0.009 |
| Week V ± SD | 0.57 ± 0.94 | 1.78 ± 1.96 | 0.010 |
| Week VI ± SD | 0.41 ± 0.79 | 1.67 ± 1.95 | 0.005 |
| Week VII ± SD | 0.44 ± 0.77 | 1.58 ± 1.95 | 0.019 |
| Week VIII ± SD | 0.24 ± 0.55 | 1.34 ± 1.82 | 0.008 |
| The mean depth reduction of the ulcer ± SD | |||
| Week I ± SD | 0.61 ± 0.81 | 0.66 ± 1.03 | 0.859 |
| Week II ± SD | 0.41 ± 0.70 | 0.63 ± 1.03 | 0.150 |
| Week III ± SD | 0.33 ± 0.63 | 0.47 ± 0.78 | 0.030 |
| Week IV ± SD | 0.24 ± 0.50 | 0.43 ± 0.65 | 0.018 |
| Week V ± SD | 0.18 ± 0.35 | 0.36 ± 0.65 | 0.049 |
| Week VI ± SD | 0.08 ± 0.25 | 0.28 ± 0.47 | 0.001 |
| Week VII ± SD | 0.04 ± 0.13 | 0.24 ± 0.48 | 0.006 |
| Week VIII ± SD | 0.01 ± 0.03 | 0.18 ± 0.46 | 0.003 |
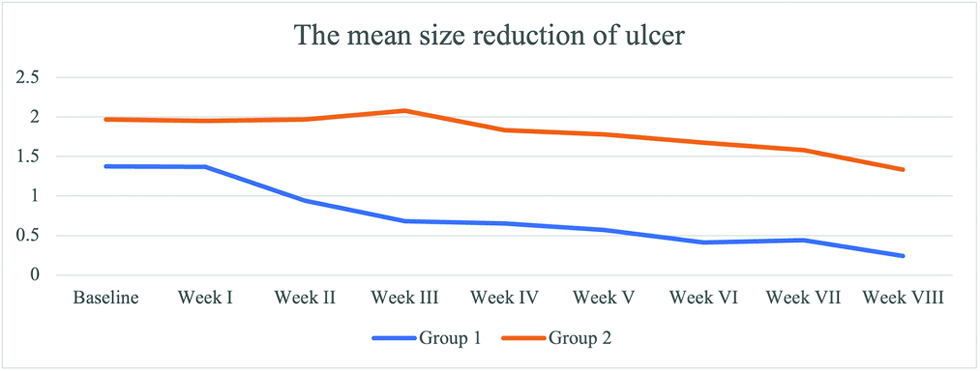
- The weekly mean size reduction of ulcers between two groups

- The weekly mean depth reduction of ulcers between two groups
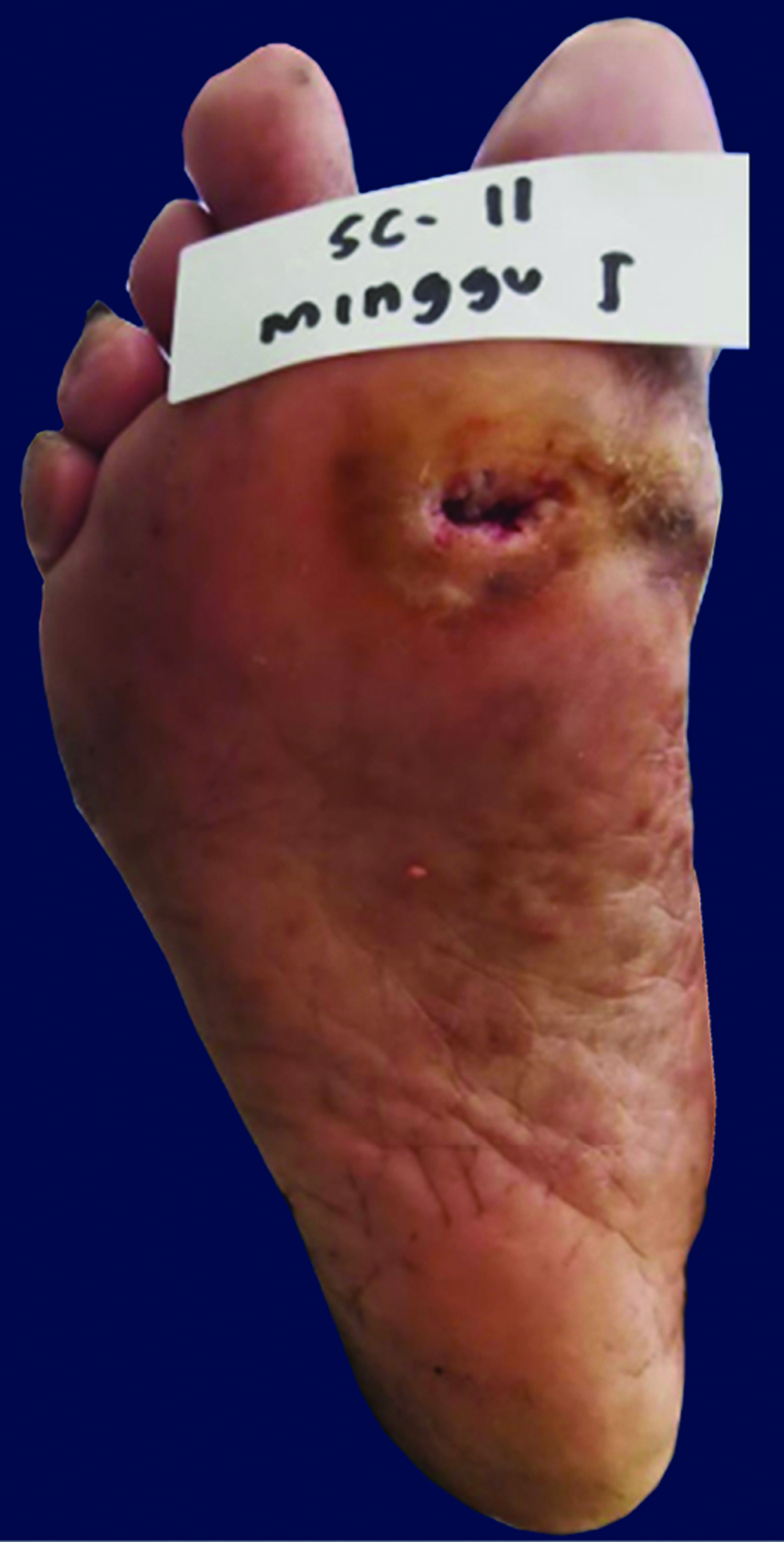
- Chronic ulcer before application in group 1
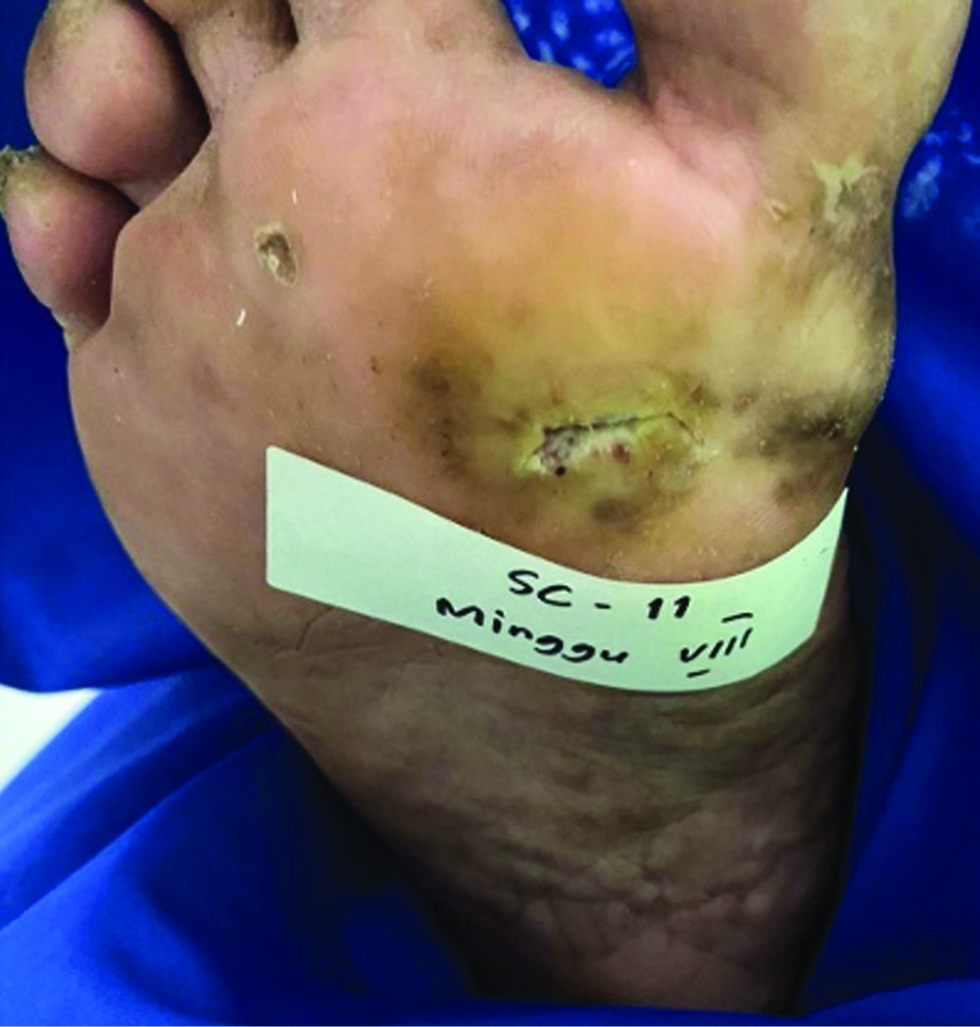
- Recovered ulcer at week 8 after gel application

- Chronic ulcer before application in group 2
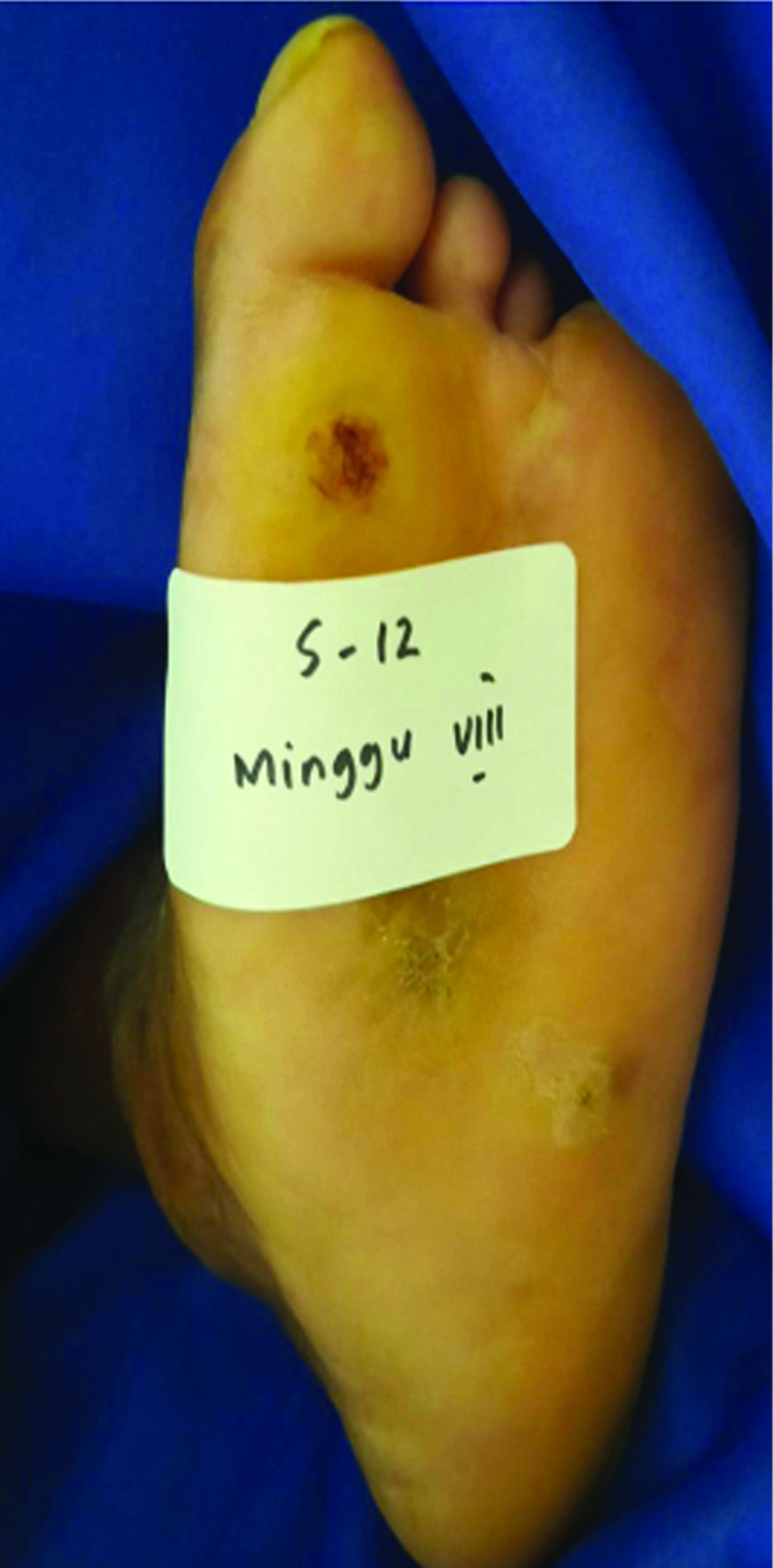
- Recovered ulcer at week 8 after application
Intention to treat analysis
The clinical improvement in the ulcers at the end of the study for group 1 was remarkable. Between group 1 and 2, the number of patients in whom ulcers were fully healed (complete closure) was 10 patients (62.5%) vs. 4 patients (25.0%) and improved (ulcer size was smaller at the end compared with that at baseline) was 6 patients (37.5%) vs. 12 patients (75.0%), respectively.
Comparison between group 1 and 2 pre-and post-intervention based on immunohistochemistry and microscopic analysis is listed in Tables 3 and 4 and Figures 6 and 7. Within group 1, cells of macrophages, fibroblasts and vascularity value were statistically significant (P < 0.05), while not in group 2. Comparison between group 1 and group 2 pre-intervention showed no significant difference in the parameters analysed (P > 0.05), whereas post-intervention showed a significant difference among macrophages, fibroblasts and vascularity value (P < 0.05).
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Pre-intervention | Post-intervention | P | Pre-intervention | Post-intervention | P | |
| Pre-intervention | Post-intervention | P | Pre-intervention | Post-intervention | P | |
| Immunohistochemistry | ||||||
| TGF-β | 6.81 ± 3.49 | 9.62 ± 4.53 | 0.155 | 6.38 ± 3.48 | 7.63 ± 4.27 | 0.422 |
| Microscopic analysis | ||||||
| Macrophages | 7.00 ± 4.08 | 11.25 ± 3.64 | 0.018 | 6.19 ± 2.01 | 6.19 ± 4.32 | 1.000 |
| Fibroblasts | 4.50 ± 2.48 | 9.81 ± 2.34 | 0.000 | 5.00 ± 2.22 | 6.81 ± 3.97 | 0.143 |
| Vascularity | 4.81 ± 1.91 | 8.25 ± 1.81 | 0.000 | 5.06 ± 2.52 | 5.63 ± 2.68 | 0.587 |
TGF-β: Transforming growth factor-β
| Pre-intervention | Post-intervention | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | P | Group 1 | Group 2 | P | |
| Group 1 | Group 2 | P | Group 1 | Group 2 | P | |
| Immunohistochemistry | ||||||
| TGF-β | 6.81 ± 3.49 | 6.38 ± 3.48 | 0.725 | 9.62 ± 4.53 | 7.63 ± 4.27 | 0.209 |
| Microscopic analysis | ||||||
| Macrophages | 7.00 ± 4.08 | 6.19 ± 2.01 | 0.483 | 11.25 ± 3.64 | 6.19 ± 4.32 | 0.001 |
| Fibroblasts | 4.50 ± 2.48 | 5.00 ± 2.22 | 0.552 | 9.81 ± 2.34 | 6.81 ± 3.97 | 0.016 |
| Vascularity | 4.81 ± 1.91 | 5.06 ± 2.52 | 0.754 | 8.25 ± 1.81 | 5.63 ± 2.68 | 0.003 |
TGF-β: Transforming growth factor-β
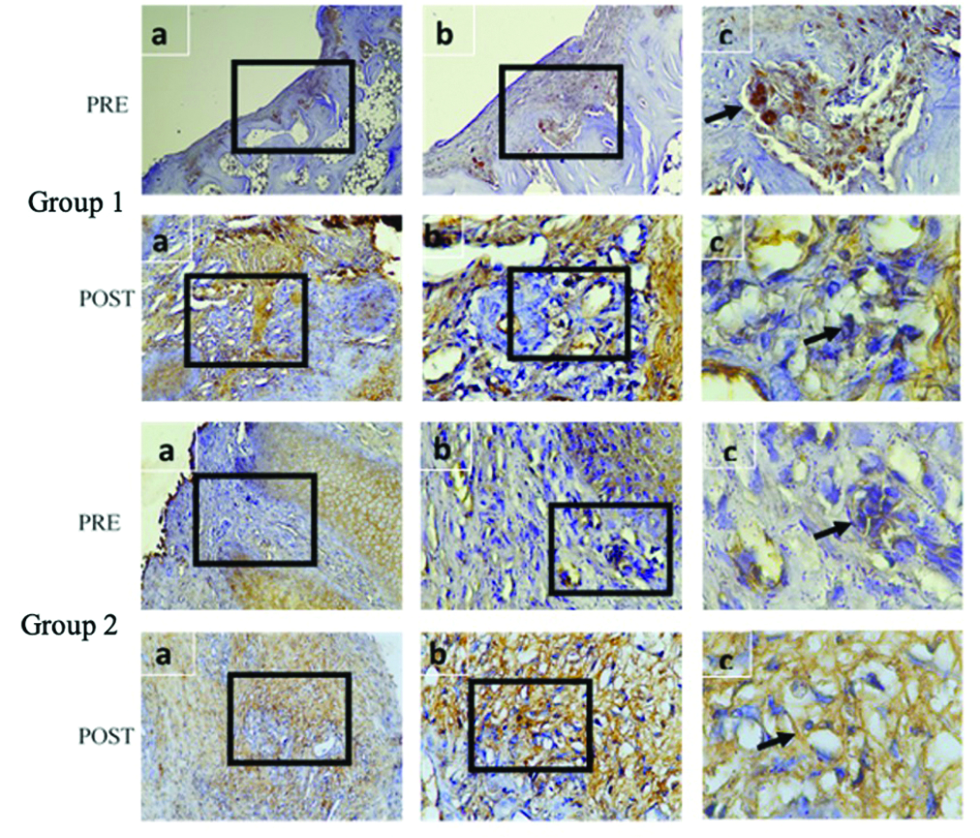
- Comparison between group 1 and 2 based on immunohistochemistry of TGF-β
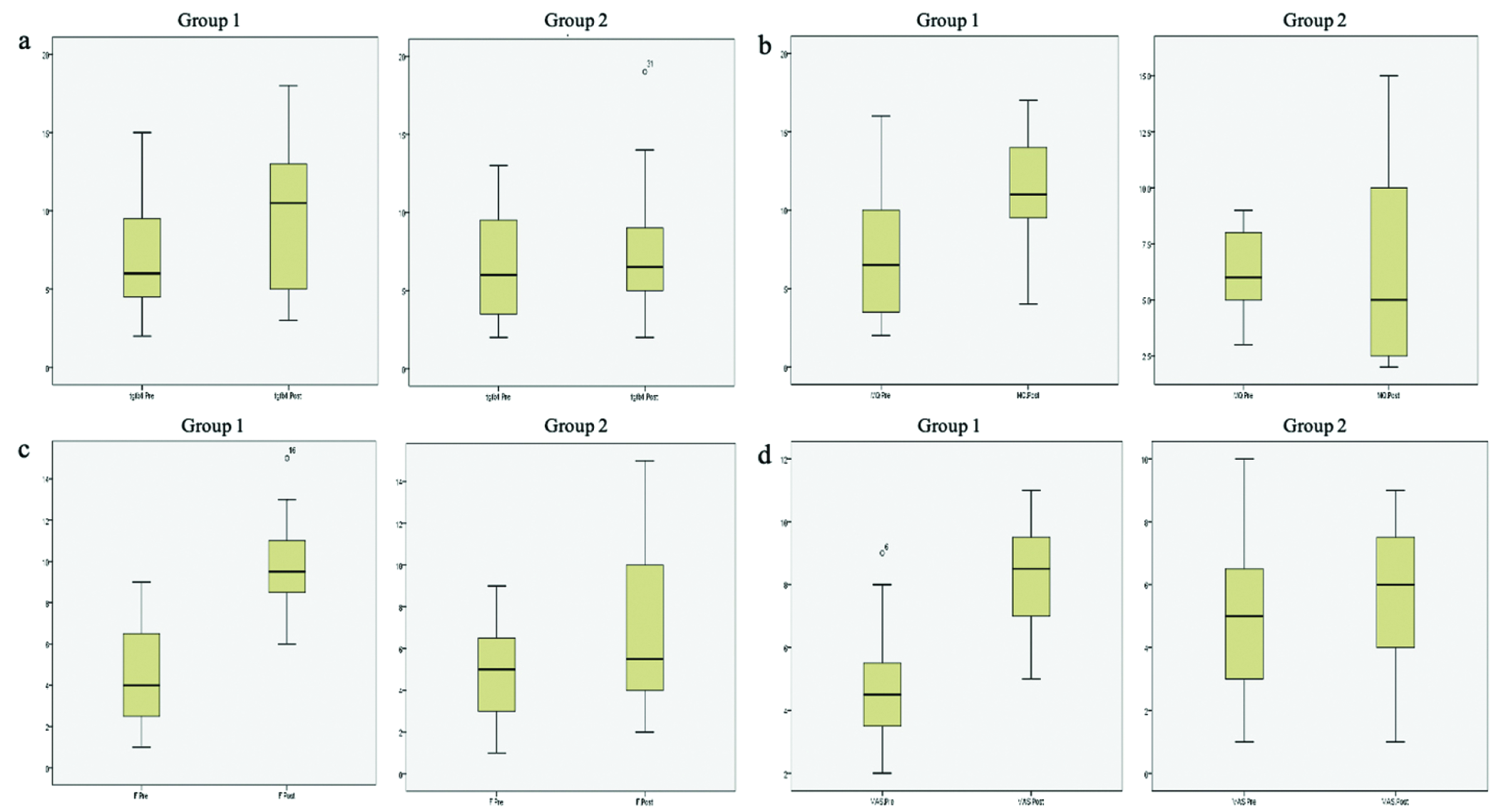
- Boxplot comparison between group 1 and 2 pre- and post-intervention on (a) TGF-β, (b) macrophages, (c) fibroblasts and (d) vascularity
Adverse reactions
No adverse reactions were encountered in any group. During clinical examination, no patient had any clinical signs of allergic contact dermatitis or infection (erythema of the skin around the ulcer, swelling, warmth on palpation, odour and exudation were absent).
DISCUSSION
Leprosy is a chronic granulomatous curable infection caused by Mycobacterium leprae infecting mucocutaneous tissues and peripheral nerves, leading to loss of sensation on the skin.15,16 The trophic ulcer, or chronic plantar ulcer, of leprosy refers to the dreaded, recalcitrant disability of the sensory-deficient foot. The chronic plantar ulcer of leprosy is one of the most common complications on the foot of leprosy patients.3,4 The pathogenesis of pressure injuries is multifactorial. Risk factors include pressure, friction, moisture, advanced age, malnutrition, diabetes, dehydration and vascular disease.17
In leprosy, these factors are further aggravated due to impairment of tactile and proprioceptive sensory disturbance, paresis, balance impairments, autonomic nerve disorder, disorder of vision, joint, toe and limb deformity leading to ulcer formation.18 With improper wound treatment or a delay in the diagnosis, the condition could subsequently lead to the formation of a chronic ulcer.
A chronic ulcer is a wound that failed to go through the physiological process in an orderly and timely manner to regain anatomic and functional integrity of the injured site or is defined as a wound that fails to heal within three months, typically showing a continuous inflammatory state with an increased presence of neutrophils as the typical biological marker.6,19
Table 1 shows that the subjects recruited were not different among the groups. At the end of the study, satisfactory outcomes were obtained in both groups. There was a trend for decreasing ulcer size and depth in both the groups [Table 2]. In the comparative test of the ulcer size between group 1 and 2, the mean width was significantly different from week two to eight of the study (P = 0.008, 0.009, 0.010, 0.005, 0.019 and 0.008, respectively). In the aspect of ulcer depth between group 1 and 2, the mean depth was also significantly different from week three to eight of the study (P = 0.030, 0.018, 0.049, 0.001, 0.006 and 0.003, respectively).
A recent randomised-controlled, single-blind, multicentre study examined the potential of allogeneic adipose mesenchymal stem cells sheets for treating diabetic foot ulcers in 39 patients. The subjects were divided into control (n = 17) and receiving allogeneic adipose mesenchymal stem cells sheet (n = 22) groups and received treatment for a maximum of 12 weeks. At the end of week 8 and week 12, ulcer closure in the allogeneic adipose mesenchymal stem cells sheet group was higher compared to the control group (73% vs. 47%, P = 0.102 and 82% vs. 53%, P = 0.053, respectively). A shorter mean time required for complete wound closure was noted (40.8 ± 5.3 days vs. 51.2 ± 3.9 days, respectively). Significantly shorter Kaplan-Meier median time (28.5 days and 63.0 days) together with a higher rate of wound size reduction at week one in the treatment group compared to the control group were also noted (49.6 ± 25.7% vs. 23.0 ± 32.2%, P = 0.007, respectively).20
This result was comparable with that of our study. The adipose mesenchymal stem cells, together with the secretomes secreted, are thought to relieve tissue stress and reduce the prolonged inflammatory phase. Compared to normally healing wounds, chronic wounds exhibit an increased level of proinflammatory cytokines, proteases, reactive oxygen species and senescent cells and a depleted population of stem cells. After an injury, microorganisms and platelet-derived growth factors, such as TGF-β or extracellular matrix molecules, recruit immune cells, which further strengthen the release of proinflammatory cytokines persisting in chronic wounds. These immune cells in the wound produce reactive oxygen species, which further damages the extracellular matrix proteins, activating additional proteases and inflammatory cytokines.6
Another study analysing the use of bone marrow-mesenchymal stem/stromal cells for non-healing ulcers of the lower extremity in humans has shown that preimplantation of mesenchymal stem/stromal cell microscopy sections show few dermal cells and extensive fibrosis, consistent with scar formation. There was a paucity of inflammatory cells, which is a consistent feature of a non-healing wound. Yet, three days after mesenchymal stem/stromal cell implantation, microscopy sections showed abundant chronic inflammatory cells, mostly polymorphonuclear cells, along with new capillary bed formation and eight days after implantation, microscopy sections showed inflammatory cells and capillary proliferation.21 The mechanisms of mesenchymal stem/stromal cell-based wound therapy have not been fully discovered, yet two mechanisms are generally postulated, (1) direct differentiation into skin cells and (2) secretion of soluble factors, such as growth factors, immune factors, chemokines and exosomes, predominantly in a paracrine manner.19
One of the unique characteristics of adipose mesenchymal stem cells in wound healing is the cellular plasticity of these cells. In vivo, adipose mesenchymal stem cells can differentiate into fibroblasts by exhibiting the morphological similarity to fibroblasts and expression of vimentin and fibronectin and heat shock protein 47.22 In our study, fibroblasts have shown to increase significantly pre- and post-intervention within and across groups (P < 0.001 and P < 0.05, respectively). It is known that the tissue repair process involves several growth factor families, such as epidermal growth factor, fibroblast growth factor, TGF-β and platelet-derived growth factor, all of which are partly produced by fibroblasts.23 Nevertheless, adipose mesenchymal stem cells were found to be not significant in increasing the TGF-β pre- and post-intervention (P = 0.155 and P = 0.725, respectively). In our study, adipose mesenchymal stem cell-conditioned medium produced epidermal growth factor, fibroblast growth factor, TGF-β and platelet-derived growth factor. Two studies using prostatic stromal cells and human bone marrow-mesenchymal stem/stromal cells found that low doses of TGF-β1 (≤ 0.25 ng/mL) increased mesenchymal stem/stromal cells proliferation, while higher doses of TGF-β1 (≥ 1 ng/mL) inhibited their proliferation.24,25 These data suggested that TGF-β could exert a biphasic, dose-dependent effect on mesenchymal stem/stromal cells, potentially explaining the contradictory results between TGF-β and mesenchymal stem/stromal cells proliferation.26
With the immunomodulatory activities of mesenchymal stem/stromal cells, hyper-inflammation of chronic wounds could be overcome, which promotes the transition of wound healing from the inflammatory phase to the proliferative phase. When activated by inflammatory factors, mesenchymal stem/stromal cells become immunosuppressive, secreting soluble mediators (i.e., nitric oxide and prostaglandin E2) which decrease excessive immune reactions.19 Mesenchymal stem/stromal cells are able to exert an effect on various immune cells (i.e., dendritic cells, natural killer, macrophages, T lymphocytes and B lymphocytes). In our study, group 1 had statistically significant results for the macrophages investigated. Macrophages within wounds are multi-functional cells with many possible phenotypes. Resident macrophages in the early healing process are able to produce multiple cytokines and chemokines stimulating the inflammatory response critical for the infiltration of leucocytes and other cells into the injured tissue. Wound macrophages are also actively phagocytic, clearing not only microbes but also apoptotic cells and necrotic materials.27
Apart from the aforementioned cellular components and growth factors for wound healing, a cellular response of angiogenesis activated by growth factors also modulate wound healing. Angiogenesis, the formation of vascularity, provides oxygen and growth factors to induce the formation of granulation tissue. Angiogenesis is stimulated by basic fibroblast growth factor, vascular endothelial growth factor (VEGF) and TGF-β signalling pathways. The major growth factors playing a role in endothelial proliferation and migration and blood vessel maturation is VEGF via mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-endothelial nitric oxide synthase (PI3K-Akt-eNOS) pathways. At the same time, one signalling pathway produces reactive oxygen species in low concentration stimulating the proliferation and migration of fibroblasts, enhancing collagen production for granulation tissue formation and wound closure. Granulation tissue formation and type III collagen are promoted principally by basic fibroblast growth factor and TGF-β and provide the structure for fibroblast and keratinocyte migration and vascular formation.28
A nonhealing ulcer (caused by diabetes) has shown defective new blood vessel formation as a contributor to a chronic wound. In a recent animal model study, it was reported that the proangiogenic factor VEGF was higher in diabetic wounds of the adipose mesenchymal stem cells + plasma-rich platelet compared to other groups, indicating that adipose mesenchymal stem cells + plasma-rich platelet stimulated angiogenesis.22 This is further reinforced by an animal model study that reported that both adipose mesenchymal stem cells and secretome increased the deposition of VEGF in the recipient wounds; specifically, more VEGF was deposited in the secretome-treatment group.29
Limitations
The present study had a few limitations. We did not perform off-loading, i.e., reducing or removing the load on the legs (bed rest or utilisation of crutches, wheelchairs, walkers and special footwear). Optimal recommendations for standing or walking activities reduction were advised, yet it was very difficult to implement due to the occupational requirement of each subject. Future experimental studies may investigate the concentration of adipose mesenchymal stem cell-conditioned medium and different methods of application correlating with the healing of the ulcer.
CONCLUSION
The adipose mesenchymal stem cell proved to be more beneficial, clinically and statistically for mean ulcer size and depth reduction than the control group. Difference in the beneficial effects between groups was seen from week two and week three to week eight, which was faster in group 1 for mean ulcer size and depth reduction. There were no complications or adverse reactions observed due to the intervention in any subject. Further research using different methods of application is recommended.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Management of ulcers in neurologically impaired feet in leprosy affected persons. Surgical reconstruction and rehabilitation in leprosy and other neuropathies. 2004;15:193-226.
- [Google Scholar]
- Interventions for skin changes caused by nerve damage in leprosy (Review) Cochrane Database Sys Rev. 2019;2019:CD004833.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidities associated with non-healing of plantar ulcers in leprosy patients. PLoS Negl Trop Dis. 2020;14:e0008393.
- [CrossRef] [PubMed] [Google Scholar]
- Trophic skin ulceration in leprosy: Evaluation of the efficacy of topical phenytoin sodium zinc oxide paste. Int J Dermatol. 2014;53:873-8.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19:575-85.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen Med. 2019;14:213-30.
- [CrossRef] [PubMed] [Google Scholar]
- Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. 2019;10:274.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. 2018;13:e0195891.
- [CrossRef] [PubMed] [Google Scholar]
- First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539-40.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Crohn’s-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: A phase I-IIa clinical trial. Stem Cells Transl Med. 2016;5:1441-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci. 2020;21:7038.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcer in leprosy: A randomized control trial. J Dermatolog Treat. 2018;29:835-40.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy. In: Fitzpatrick’s Dermatology (9th ed.). New York (United States of America): McGraw Hill; 2019. p. :2892-924.
- [Google Scholar]
- Bacteriology of Leprosy. In: IAL Textbook of Leprosy (>2nd ed.). New Delhi (India): The Health Sciences Publisher; 2017. p. :90-104.
- [Google Scholar]
- Beyond skin deep: Managing pressure injuries. JAAPA. 2018;31:10-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between plantar pressure and sensory disturbance in patients with Hansen’s disease- Prelimenary research and review of the literature. Sensors (Basel). 2020;20:6976.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal stem cells for chronic wound healing: Current status of preclinical and clinical studies. Tissue Eng Part B Rev. 2020;26:555-70.
- [CrossRef] [PubMed] [Google Scholar]
- Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes. 2019;68:837-46.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359-66.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res Ther. 2021;12:226.
- [CrossRef] [PubMed] [Google Scholar]
- Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12:735.
- [CrossRef] [PubMed] [Google Scholar]
- Dual regulation of proliferation and growth arrest in prostatic stromal cells by transforming growth factor-beta1. Endocrinology. 2003;144:4280-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of TGF-β1 on bone marrow stem cells proliferation, osteogenic differentiation and maturation. Regen Res. 2012;1:20-6.
- [Google Scholar]
- TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine and Growth Factor Rev. 2018;43:25-37.
- [CrossRef] [PubMed] [Google Scholar]
- Macrophages in healing wounds: Paradoxes and paradigms. Int J Mol Sci. 2021;22:950.
- [CrossRef] [PubMed] [Google Scholar]
- Rational selection of bioactive principles for wound healing applications: Growth factors and antioxidants. Int Wound J 2021:1-14.
- [CrossRef] [PubMed] [Google Scholar]
- Adipose tissue-derived mesenchymal stem cells (ADMSCs) and ADMSC-derived secretome expedited wound healing in a rodent model - A preliminary study. Clin Cosmet Investig Dermatol. 2021;14:753-64.
- [CrossRef] [PubMed] [Google Scholar]






