Translate this page into:
The molecular fingerprint of human papillomavirus infection and its effect on the Langerhans cell population in squamous cell carcinomas of the genital skin
2 Postgraduate studies and Research Section, Superior School of Medicine, Instituto Politecnico Nacional, Mexico City, Mexico,
3 Dermatopathology Service, Mexico's General Hospital "Dr. Eduardo Liceaga", Mexico City, Mexico,
4 Department of Human Microbiology, University of Panama and Department of Parasitology, Gorgas Memorial Institute of Health Studies, Panama,
Correspondence Address:
Jose M Rios-Yuil
Cl�nica Hospital San Fernando, Las Sabanas, V�a Espa�a, Ciudad de Panam�, Panam�
| How to cite this article: Rios-Yuil JM, Herrera-Gonzalez NE, Aguilar-Faisal JL, Lara-Padilla E, Mercadillo-Perez P, Moreno-Lopez LM, Marquez-Ramirez AK, Saldana-Patino A, Rubio-Gayosso I. The molecular fingerprint of human papillomavirus infection and its effect on the Langerhans cell population in squamous cell carcinomas of the genital skin. Indian J Dermatol Venereol Leprol 2014;80:381 |
Abstract
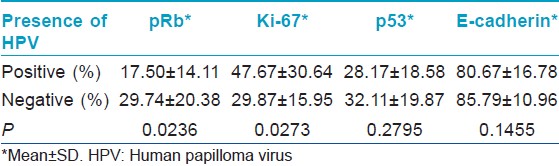
Background: Information is scarce about the presence of molecular alterations related to human papillomavirus (HPV) infection in squamous cell carcinomas of the genital skin and about the effect of this infection in the number of Langerhans cells present in these tumors. Aims: To determine the presence of HPV in genital skin squamous cell carcinomas and to see the relationship between HPV infection and changes in the expression of Ki-67 antigen (Ki-67), p53 protein (p53), retinoblastoma protein (pRb) and E-cadherin and to alterations in Langerhans cell density, if any. Methods: A descriptive, comparative, retrospective and cross-sectional study was performed with all the cases diagnosed as squamous cell carcinomas of the genital skin at the Dermatopathology Service from 2001 to 2011. The diagnosis was verified by histopathological examination. The presence of HPV was examined using chromogenic in situ hybridization, and protein expression was studied via immunohistochemical analysis. Results: The 34 cases studied were verified as squamous cell carcinomas and 44.1% were HPV positive. The degree of expression of pRb was 17.50% ±14.11% (mean ± SD) in HPV-positive cases and 29.74% ±20.38% in HPV-negative cases (P = 0.0236). The degree of expression of Ki-67 was 47.67% ±30.64% in HPV-positive cases and 29.87% ±15.95% in HPV-negative cases (P = 0.0273). Conclusion: HPV infection was related to lower pRb expression and higher Ki-67 expression in comparison with HPV negative samples. We could not find a relationship between HPV infection and the degree of expression of p53 and E-cadherin or with Langerhans cell density.INTRODUCTION
The effect of human papillomavirus (HPV) infection on cell cycle regulatory proteins (CCRP) has been related to the development of squamous cell carcinomas (SCC) in mucosal surfaces; however the few studies about this relationship in genital skin show contradictory results. [1],[2] There is even less information available about the presence of HPV and the number of Langerhans cells (LC) present in these neoplasms. None of these studies have been performed on penile skin. [3] Therefore, we designed a study to determine if HPV was related to changes on CCRP and E-cadherin in neoplastic cells and to alterations in Langerhans cell density in genital skin SCC.
METHODS
Type of study and case selection
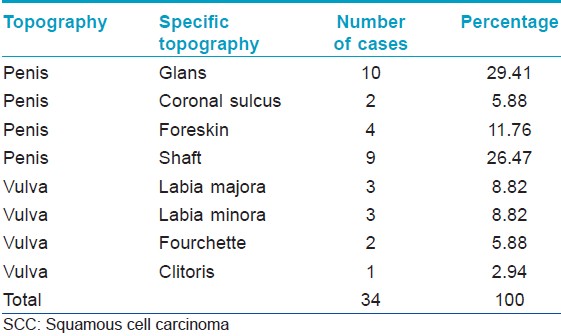
A descriptive, observational, retrospective, cross-sectional and comparative study was performed. We examined every skin biopsy that was diagnosed as genital skin SCC (penile and vulvar) by the Dermatopathology Service between 2001 and 2011. Genital skin biopsies that did not have a definitive histopathological diagnosis of SCC, that did not have paraffin-embedded tissue in the archives, or that did not have enough remaining tissue in the paraffin blocks were excluded. The study variables were sex, age, topography, presence of genital skin SCC (penile or vulvar), presence of HPV, degree of expression of p53, pRb, ki-67 and E-cadherin, and Langerhans cell density. The whole tumor present in the slides was analyzed with a conventional dual head light microscope (Carl Zeiss, Jena, Germany) and pictures were taken with a Canon PowerShot G5 Digital Camera (Canon Inc., Yokohama, Japan).
Histological analysis
In order to confirm the presence of genital skin SCC, two slides were sectioned from each paraffin block and were stained with hematoxylin and eosin.
In situ hybridization
The presence of HPV was determined with chromogenic in situ hybridization (CISH) using a ZytoFast HPV DNA Probe that detects HPVs 16, 18, 31, 33 and 35 (ZytoVision GmbH, Bremerhaven, Germany) and the ZytoFast CISH implementation Kit AP-NBT/BCIP (ZytoVision GmbH, Bremerhaven, Germany) following the manufacturer′s instructions with a few modifications. Briefly, 5 μm sections of formalin-fixed, paraffin-embedded tissues underwent deparaffinization, heat-induced target retrieval in EDTA buffer and proteinase K digestion. Slides were hybridized with the previously mentioned biotinylated probe. Probe binding was detected using a streptavidin-alkaline phosphatase conjugate and a nitro-blue tetrazolium chloride (NBT)/5-bromo-4- chlo ro-3′- indoly phosphate (BCIP) chromogen. Nuclear Fast red was used as counterstain (Manufacturing Chemists, Cincinnati, OH).
Immunohistochemistry
The effect of HPV infection on CCRP was studied by immunohistochemical staining for p53 (Clone DO7, Bio SB Inc., Santa Barbara, CA), pRb (Clone SPM353, Bio SB Inc., Santa Barbara, CA), ki-67 (Clone SP6, Bio SB Inc., Santa Barbara, CA) and E-cadherin (Clone EP700Y, Bio SB Inc., Santa Barbara, CA) following the manufacturer′s instructions. The expression degree of each marker was calculated as the percentage of neoplastic cells that expressed the immunohistochemical staining in the nucleus (p53, pRb, Ki-67) or in the cell membrane (E-cadherin). In order to determine this percentage, we counted the positive cells present in the whole tumor. The amount of high-power fields counted varied according to variations in tumor size. We counted an average of 21 high-power fields. After obtaining the degree of expression of CCRP for each case, we calculated an average for all cases and then we calculated an average for HPV-positive and HPV-negative cases. We also determined if the pattern of staining was uniform or patchy.
Langerhans cells (LC) were identified by immunohistochemical staining for CD1a antigen using a rabbit monoclonal antibody (Clone EP80, Bio SB Inc., Santa Barbara, CA) following the manufacturer′s instructions. The whole tumor present in the slide was analyzed. LC was identified by the presence of the immunohistochemical staining in the membrane and cytoplasm of cells with dendritic morphology. LC density was calculated as the mean number of LC present per high-power field of neoplastic tissue. As described before, we counted an average of 21 high-power fields. After obtaining the LC density for each case, we calculated an average for all HPV-positive cases and for all HPV-negative cases.
Statistical analysis
The statistical analysis was performed with Epi Info v. 3.4.3© , and we determined absolute or relative (percentage) frequencies for the variables. Data were expressed as mean and standard deviation. The statistical significance was determined with the independent Student t-test. A P ≤ 0.05 was considered statistically significant.
Ethical considerations
This protocol was approved by the Review Board and Ethics Committee of our Hospital. We followed the principles of the Helsinki Declaration of 1975 with the modifications of 1993, the International Ethical Guidelines for Biomedical Research involving Human Subjects and the Mexican General Health Law.
RESULTS
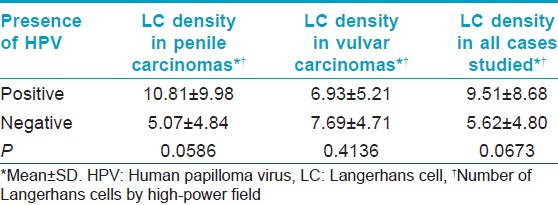
Thirty-four patients were studied, 73.5% were male and the median age was 52 years (range: 23-91 years). The glans penis was the most commonly affected area [Table - 1]. None of the patients had associated warts. The evolution of the lesions was not specified. All cases were verified as SCC through histopathological analysis [Figure - 1]. The degree of expression of p53 (30.37% ±19.12%), pRb (24.34% ±18.68%), ki-67 (37.72% ±24.85%) and E-cadherin (83.53% ±13.85%) and the mean LC density (7.34 ± 6.96) were determined in all cases and an average for each marker was calculated. HPV was present in 44.1% of the cases studied with CISH [Figure - 2]. pRb expression was reduced in HPV-positive cases; while Ki-67 expression [Figure - 3] was increased in HPV-positive cases [Table - 2]. We could not determine if there was a relationship between HPV infection and p53 expression or E-cadherin expression [Table - 2]. We could not find a relationship between HPV infection and LC density, not even after analyzing vulvar and penile cancers separately [Figure - 4] [Table - 3]. The pattern of staining was uniform in immunohistochemistry.
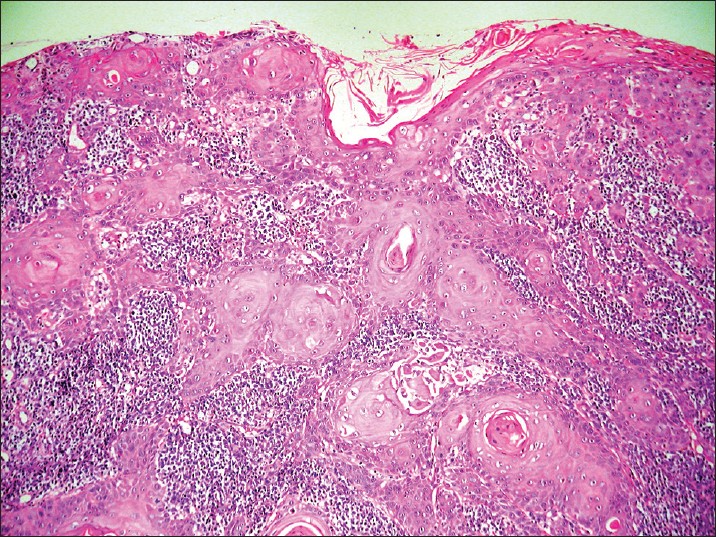 |
| Figure 1: Well-differentiated squamous cell carcinoma of the vulva, representative case (H and E, ×40) |
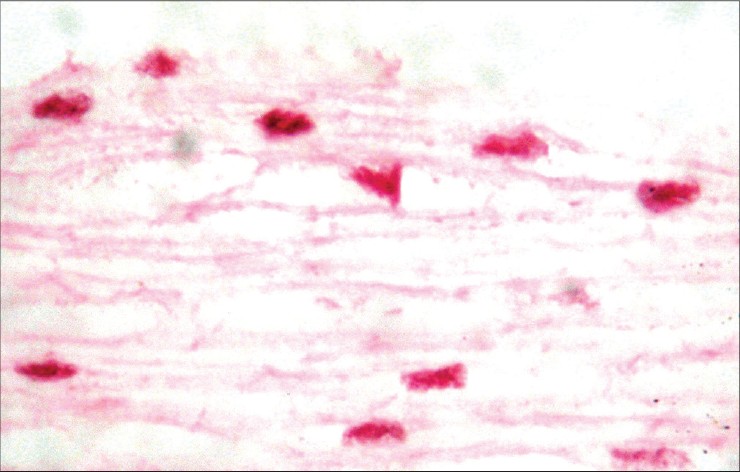 |
| Figure 2: Chromogenic in situ hybridization showing nuclear positivity in HPV infected keratinocytes (×400) |
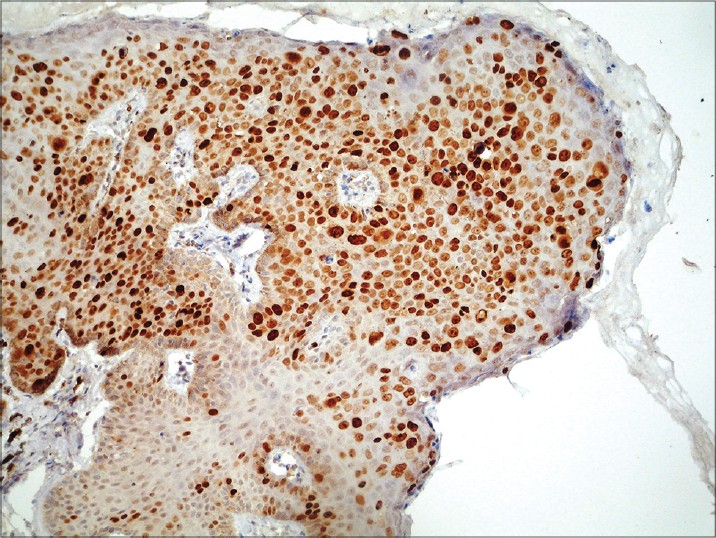 |
| Figure 3: Immunohistochemistry for Ki-67: approximately 75% of neoplastic cells show nuclear positivity (×100) |
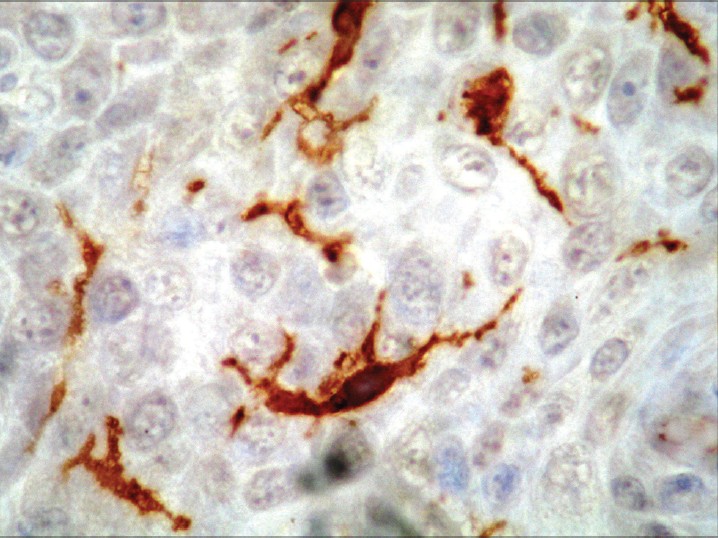 |
| Figure 4: Immunohistochemistry for CD1a: positivity is observed in the membrane and cytoplasm of Langerhans cells (×400) |
The mechanisms that explain the development of SCC of the genital skin are not well understood. By analogy to what has been shown in mucosal epithelia, HPV infection has been proposed as one of the factors that could lead to the development of genital skin SCC. HPV are small (55 nm) DNA viruses that infect the epithelial cells of the skin and mucous membranes. [4],[5],[6],[7],[8],[9],[10],[11],[12] The oncogenic role of some HPV types like HPV-16 has been clearly established in several mucous membrane cancers such as cervical carcinoma. [13] Approximately 80-90% of HPV-positive vulvar cancers are infected with HPV-16, 18, 31, 33 or 35. [7] Several pathogenic mechanisms could explain the relationship between mucosal HPV infection and the development of SCC. E5, E6 and E7 are potent HPV oncogenes that promote cell cycle dysregulation, increased proliferation and survival of malignant cells. [6],[10],[14],[15],[16],[17],[18],[19] HPV may also play a role in the development of SCC by reducing local immune surveillance. Several studies have linked HPV infection of mucosal and skin epithelial cells with a reduction in the number of LC in these tissues. [3],[20],[21],[22],[23],[24],[25] Langerhans cells are dendritic cells that play a key role in immune surveillance because they are the most potent epidermal antigen-presenting cells. LC are found on a regular basis in the epithelial layers of normal skin and mucous membranes. [20] E-cadherin is one of the main adhesion molecules expressed by squamous epithelial cells and it plays a key role in the definition of tissue architecture and differentiation. It has been shown that LC express high levels of E-cadherin on their cell membranes allowing them to adhere to keratinocytes. [21]
In our study, HPV was found in 44.1% of the cases of genital skin SCC (CISH). This result is similar to other studies. Tsimplaki, et al., found that 50% of the cases of vulvar SCC and 71.4% of the cases of vulvar intraepithelial neoplasia (VIN) were HPV positive. [26] Insinga, et al., in a systematic review in the United States, found that 65.3% of vulvar SCC were HPV positive and 49.5% of the cases were caused by HPV-16. [27] Sutton, et al., found HPV in 69.8% of the cases of vulvar SCC. [28] Larsson, et al., in a study of vulvar SCC in Sweden, found HPV in 30.8% of the cases. [29]
We could not find a difference between the degree of expression of p53 in HPV-positive and HPV-negative cases. As in our study, Stankiewicz, et al. could not find a correlation between HPV infection and p53 expression in penile SCC. [2] However, studies of SCC involving other sites had different findings. Stasikowska-Kanicka, et al., in a study of sinonasal inverted papilloma and laryngeal carcinoma; [14] Zhang, et al., in head and neck squamous cell carcinoma [30] and Conesa-Zamora, et al. in precursor lesions of cervical carcinoma, [16] showed that HPV positivity was related to a relative decrease in p53 expression.
The degree of expression of pRb was 17.50% ± 14.11% (mean ± SD) in HPV-positive cases and 29.74% ± 20.38% in HPV-negative cases (P = 0.0236). Stankiewicz, et al. obtained similar results in a study of penile SCC. They found that HPV infection negatively correlated with pRb concentrations. [2] This could be explained by the capacity of the E7 oncoprotein of high risk HPVs to induce the proteasomal-mediated degradation of pRb. [16],[18]
The degree of expression of Ki-67 was 47.67% ± 30.64% in HPV-positive cases and 29.87% ± 15.95% in HPV-negative cases (P = 0.0273). Our results agree with the data obtained by Conesa-Zamora, et al. in the previously mentioned investigation of precursor lesions of cervical carcinoma. [16] Samir, et al. in a study of cervical intraepithelial neoplasia (CIN) found that HPV infection correlated with the degree of expression of Ki-67. [31] However, not every study has been able to prove this relationship. Stankiewicz, et al., in a study of verrucous carcinoma of the penis, found low levels of Ki-67 expression. This suggests that HPV infection might not be implicated in the pathophysiology of this subtype of SCC. [2]
We could not find a relationship between HPV infection and E-cadherin expression. Our results differ from the findings of other studies. Hubert, et al. found that E-cadherin expression was reduced in CIN and in cervical SCC when compared with normal exocervical epithelia. [32] Caberg, et al. showed that HPV-infected epithelial cells from cervical dysplastic lesions had lower levels of E-cadherin. [21]
We could not prove a relationship between HPV infection and LC density, not even after analyzing vulvar and penile cancers separately. We were unable to find previous studies of this relationship in penile SCC. Research on SCC of other sites has revealed contradictory results. Most of the studies have shown that HPV is associated with a reduction in LC density; however, in some of them, this density was not affected or was increased. Jiménez-Flores, et al. found that the frequency of CD1a-positive dendritic cells per area was reduced in approximately 50% in HPV-positive epithelial sheets prepared from fresh cervical biopsies. [33] Li, et al. found fewer LC in HPV-positive esophageal cancers than in HPV-negative cases. [22] Caberg, et al. demonstrated a reduction in LC in HPV-infected cervical dysplastic lesions. [21] Hubert, et al. showed that the density of CD1a-positive LC was lower in squamous intraepithelial lesions and SCC of the uterine cervix when compared with normal exocervical epithelia. [32] Conversely, Pereira, et al. found that HPV infection was related to a reduction in LC in oral SCC but this relationship did not reach statistical significance. [23] McArdle, et al. found an increase in LC density in cases with CIN when compared with normal ectocervix. [34]
The fact that HPV infection did not cause a reduction in LC density in our study could be attributed to the small number of cases available to study. It is important to state that our findings are not necessarily indicative of a normal immune response against the tumor. LC might have been present but not properly functioning. There could be a defective recognition, internalization, processing, or presentation of tumor antigens by LC, as well as a deficit in their migration to gain access to T-cells. They could also be inhibited by physical contact with regulatory T cells or by the presence of myeloid-derived suppressor cells in the tumor microenvironment. [35] LC might also be inactivated due to a lack of secretion of proinflammatory cytokines by the infected keratinocytes. It has been shown that HPV infection limits the capacity of the keratinocyte to secrete proinflammatory cytokines such as interferon α, interferon β, interleukin 1, interleukin 6, among others. [36] For these reasons, further studies are needed to address the effect that HPV infection might have on LC function in genital skin SCC.
In conclusion, HPV infection was associated with lower pRb expression and higher Ki-67 expression in comparison with HPV negative samples. We could not find a relationship between HPV infection and the degree of expression of p53 and E-cadherin or with LC density. The main limitation of this study was the small number of patients that could be included because this is not a frequent disease. We recommend additional studies with larger sample sizes including patients from other medical centers.
| 1. |
Vasiljevic N, Hazard K, Dillner J, Forslund O. Four novel human betapapillomaviruses of species 2 preferentially found in actinic keratosis. J Gen Virol 2008;89:2467-74.
[Google Scholar]
|
| 2. |
Stankiewicz E, Kudahetti SC, Prowse DM, Ktori E, Cuzick J, Ambroisine L, et al. HPV infection and immunochemical detection of cell-cycle markers in verrucous carcinoma of the penis. Mod Pathol 2009;22:1160-8.
[Google Scholar]
|
| 3. |
Nakayama Y, Asagoe K, Yamauchi A, Yamamoto T, Shirafuji Y, Morizane S, et al. Dendritic cell subsets and immunological milieu in inflammatory human papilloma virus-related skin lesions. J Dermatol Sci 2011;63:173-83.
[Google Scholar]
|
| 4. |
Jeon JH, Shin DM, Cho SY, Song KY, Park NH, Kang HS, et al. Immunocytochemical detection of HPV16 E7 in cervical smear. Exp Mol Med 2007;39:621-8.
[Google Scholar]
|
| 5. |
Zheng ZM, Baker CC. Papillomavirus genome structure, expression and post-translational regulation. Front Biosci 2006;11:2286-302.
[Google Scholar]
|
| 6. |
Munday JS, Kiupel M. Papillomavirus-associated cutaneous Neoplasia in Mammals. Vet Pathol 2010;47:254-64.
[Google Scholar]
|
| 7. |
Iftner A, Klug SJ, Garbe C, Blum A, Stancu A, Wilczynski SP, et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res 2003;63:7515-9.
[Google Scholar]
|
| 8. |
Andersson K, Waterboer T, Kirnbauer R, Slupetzky K, Iftner T, de Villiers EM, et al. Seroreactivity to cutaneous human papillomaviruses among patients with nonmelanoma skin cancer or benign skin lesions. Cancer Epidemiol Biomarkers Prev 2008;17:189-95.
[Google Scholar]
|
| 9. |
Chaudhary AK, Singh M, Sundaram S, Mehrotra R. Role of human papillomavirus and its detection in potentially malignant and malignant head and neck lesions: Updated review. Head Neck Oncol 2009;1:22.
[Google Scholar]
|
| 10. |
Feller L, Khammissa RA, Wood NH, Lemmer J. Epithelial maturation and molecular biology of oral HPV. Infect Agent Cancer 2009;4:16.
[Google Scholar]
|
| 11. |
Zhang D, Zhang Q, Zhou L, Huo L, Zhang Y, Shen Z, et al. Comparison of prevalence, viral load, physical status and expression of human papillomavirus-16, -18 and -58 in esophageal and cervical cancer: A case-control study. BMC Cancer 2010;10:650.
[Google Scholar]
|
| 12. |
Maufort JP, Williams SM, Pitot HC, Lambert PF. Human papillomavirus 16 E5 oncogene contributes to two stages of skin carcinogenesis. Cancer Res 2007;67:6106-12.
[Google Scholar]
|
| 13. |
Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst 2006;98:389-95.
[Google Scholar]
|
| 14. |
Stasikowska-Kanicka O, W¹growska-Danilewicz M, Danilewicz M. Effect of human papillomavirus on cell cycle-related proteins p16INK4A, p21waf1/cip1, p53 and cyclin D1 in sinonasal inverted papillomaand laryngeal carcinoma. An in situ hybridization study. Folia Histochem Cytobiol 2011;49:34-40.
[Google Scholar]
|
| 15. |
Tsai YY, Chang CC, Chiang CC, Yeh KT, Chen PL, Chang CH, et al. HPV infection and p53 inactivation in pterygium. Mol Vis 2009;15:1092-7.
[Google Scholar]
|
| 16. |
Conesa-Zamora P, Doménech-Peris A, Orantes-Casado FJ, Ortiz-Reina S, Sahuquillo-Frías L, Acosta-Ortega J, et al. Effect of human papillomavirus on cell cycle-related proteins p16, Ki-67, Cyclin D1, p53, and ProEx C in precursor lesions of cervical carcinoma: A tissue microarray study. Am J Clin Pathol 2009;132:378-90.
[Google Scholar]
|
| 17. |
Vasilescu F, Ceauºu M, Tãnase C, Stãnculescu R, Vlãdescu T, Ceauºu Z. p53, p63 and Ki-67 assessment in HPV-induced cervical neoplasia. Rom J Morphol Embryol 2009;50:357-61.
[Google Scholar]
|
| 18. |
Buitrago-Pérez A, Garaulet G, Vázquez-Carballo A, Paramio JM, García-Escudero R. Molecular signature of hpv-induced carcinogenesis: Prb, p53 and gene expression profiling. Curr Genomics 2009;10:26-34.
[Google Scholar]
|
| 19. |
Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, et al. p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: A systematic review and meta-analysis. Cancer Treat Rev 2009;35:210-20.
[Google Scholar]
|
| 20. |
Leong CM, Doorbar J, Nindl I, Yoon HS, Hibma MH. Loss of epidermal Langerhans cells occurs in human papillomavirus alpha, gamma, and mu but not beta genus infections. J Invest Dermatol 2010;130:472-80.
[Google Scholar]
|
| 21. |
Caberg JH, Hubert PM, Begon DY, Herfs MF, Roncarati PJ, Boniver JJ, et al. Silencing of E7 oncogene restores functionalc E-cadherin expression in human papillomavirus 16-transformed keratinocytes. Carcinogenesis 2008;29:1441-7.
[Google Scholar]
|
| 22. |
Li J, Zhang Y, Gao D. [Study on the interrelationship between human papilloma virus infection and Langerhans cell in carcinogenesis of esophagus]. Zhonghua Bing Li Xue Za Zhi 1996;25:83-5.
[Google Scholar]
|
| 23. |
Pereira KM, Soares RC, Oliveira MC, Pinto LP, Costa Ade L. Immunohistochemical staining of Langerhans cells in HPV-positive and HPV-negative cases of oral squamous cells carcinoma. J Appl Oral Sci 2011;19:378-83.
[Google Scholar]
|
| 24. |
D'Costa ZJ1, Jolly C, Androphy EJ, Mercer A, Matthews CM, Hibma MH. Transcriptional repression of E-cadherin by human papillomavirus type 16 E6. PLoS One 2012;7:e48954.
[Google Scholar]
|
| 25. |
Sperling T, O³dak M, Walch-Rückheim B, Wickenhauser C, Doorbar J, Pfister H, et al . Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog 2012;8:e1002833.
[Google Scholar]
|
| 26. |
Tsimplaki E, Argyri E, Michala L, Kouvousi M, Apostolaki A, Magiakos G, et al. Human papillomavirus genotyping and e6/e7 mRNA expression in greek women with intraepithelial neoplasia and squamous cell carcinoma of the vagina and vulva. J Oncol 2012;2012:893275.
[Google Scholar]
|
| 27. |
Insinga RP, Liaw KL, Johnson LG, Madeleine MM. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev 2008;17:1611-22.
[Google Scholar]
|
| 28. |
Sutton BC, Allen RA, Moore WE, Dunn ST. Distribution of human papillomavirus genotypes in invasive squamous carcinoma of the vulva. Mod Pathol 2008;21:345-54.
[Google Scholar]
|
| 29. |
Larsson GL, Helenius G, Andersson S, Elgh F, Sorbe B, Karlsson MG. Human papillomavirus (HPV) and HPV 16-variant distribution in vulvar squamous cell carcinoma in Sweden. Int J Gynecol Cancer 2012;22:1413-9.
[Google Scholar]
|
| 30. |
Zhang JL1, Sun Z, Huo Z, Luo YF, Ma SQ, Wang DT, et al. [Infection of human papillomavirus 16/18 DNA in patients with head and neck squamous cell carcinoma and its relationship withexpression of Ki-67 and P53 protein]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010;32:429-32.
[Google Scholar]
|
| 31. |
Samir R, Asplund A, Tot T, Pekar G, Hellberg D. High-Risk HPV Infection and CIN Grade Correlates to the Expression of c-myc, CD4+, FHIT, E-cadherin, Ki-67, and p16INK4a. J Low Genit Tract Dis 2011;15:280-6.
[Google Scholar]
|
| 32. |
Hubert P, Caberg JH, Gilles C, Bousarghin L, Franzen-Detrooz E, Boniver J, et al. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre) neoplastic lesions. J Pathol 2005;206:346-55.
[Google Scholar]
|
| 33. |
Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Muñoz-Molina R, Balderas-Carrillo O, de la Luz Diaz-Soberanes M, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology 2006;117:220-8.
[Google Scholar]
|
| 34. |
McArdle JP, Muller HK. Quantitative assessment of Langerhans' cells in human cervical intraepithelial neoplasia and wart virus infection. Am J Obstet Gynecol 1986;154:509-15.
[Google Scholar]
|
| 35. |
Fujita H, Suárez-Fariñas M, Mitsui H, Gonzalez J, Bluth MJ, Zhang S, et al. Langerhans cells from human cutaneous squamous cell carcinoma induce strong type 1 immunity. J Invest Dermatol 2012;132:1645-55.
[Google Scholar]
|
| 36. |
Stanley MA. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin Microbiol Rev 2012;25:215-22.
[Google Scholar]
|
Fulltext Views
3,341
PDF downloads
2,808





