Translate this page into:
The rainbow pattern in dermoscopy: A panorama of Kaposi’s and non-Kaposi’s lesions
Corresponding author: Dr. Zineb Bennouna, Department of Dermatology, Hassan II University Hospital Fez, Fez, Morocco. zineb.bennouna@usmba.ac.ma
-
Received: ,
Accepted: ,
How to cite this article: Bennouna Z, Soughi M, Douhi Z, Elloudi S, Baybay H, Mernissi F. The rainbow pattern in dermoscopy: A panorama of Kaposi’s and non-Kaposi’s lesions. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1381_2024
Dear Editor,
The term rainbow was initially attached to Kaposi’s sarcoma (KS) and considered a very specific dermoscopic sign of this disease. However, its diagnostic significance has been debated in recent years because of its presence in other lesions. In this study, we prospectively analysed 500 lesions, including inflammatory, tumoural dermatoses, and vascular lesions, over a period of 2 years. All lesions were examined using the dermoscope dermlite 4 with polarised and non-polarised light. Lesions were classified as Kaposi’s and non-Kaposi’s lesions. All tumours were confirmed histologically. Among the 500 lesions examined, 69 presented a rainbow pattern (RP) on dermoscopy. There were 18 (26,08%) Kaposi’s lesions and 51(73,91%) non-Kaposi’s lesions. RP was found in both cutaneous (n=18) and nail (n=2) involvement of KS. In non-Kaposi’s lesions, this pattern was found in these pathologies: scleroatrophic scars (n=24, 34.78%), lichen planus (n=5, 7.24%), angiokeratoma (n=4, 5.79%), pyogenic granuloma (n=3, 4.34%), nodular basal cell carcinoma (BCC) (n=3, 4.34%), squamous cell carcinoma (SCC) (n=2, 2.89%), melanoma (n=2, 2.89%), cystic lymphangioma (n=1, 1.44%), glomus tumour (n=1, 1.44%), keratoacanthoma (n=1, 1.44%), extra genital scleroatrophic lichen (n=1, 1.44%), pigmented epithelioid melanocytoma (n=1, 1.44%), Ewing sarcoma (n=1, 1.44%), dermatofibrosarcoma protuberans (n=1, 1.44%), and cutaneous metastasis of breast cancer (n=1, 1.44%). In BCC, RP was seen in both pigmented and non-pigmented lesions. In all these lesions, this pattern was seen only under polarised light. All lesions were infiltrated and presented as papules, plaques, nodules, or tumours. They were most commonly localised in the limb (n=49, 71.01%), followed by the cephalic region (n=7, 10.14%). Regarding the distribution of the RP in lesions, a clear predominance of multifocal distribution was observed in vascular and non-vascular lesions. The RP alone could not establish the clinical diagnosis; other dermoscopic signs were collected to allow a diagnosis of certain lesions, and others required histological confirmation. Dermoscopic findings and the corresponding histology have been summarised in Table 1 and in Figures 1-3.
| Diagnosis | Number of cases | Distribution of RP | Dermoscopy | Histological confirmation |
|---|---|---|---|---|
| KS | 18 | multifocal | RP, white lines, scale, purple- red colour | Tumour proliferation made of spindle cells dissociated by numerous congested vascular cavities |
| Scleroatrophic scars | 24 | multifocal | RP, erythematous background, white areas, peripheral pigmentation | - |
| Lichen planus | 5 | multifocal | RP, scales, Wickham striae |
2 cases. Saw-toothed rete ridges, band-like lympho-histiocytic infiltrate at the dermo-epidermal junction. Wedge-shaped hypergranulosis Civatte bodies |
| Angiokeratoma | 4 | multifocal | RP, dark lacunae, erythema, red lacunae and haemorrhagic crusts |
Vascular ectasia of the papillary dermis. Overlying epidermal hyperplasia characterised by acanthosis, elongation of the rete, and hyperkeratosis, with the epidermis encircling the dilated vascular spaces. |
| Pyogenic granuloma | 3 | focal | RP, crust, white collarette | lobular arrangement of capillaries |
| Nodular basal cell carcinoma | 3 | multifocal |
Non-pigmented: RP, Blue-grey ovoid nests, Arborising telangiectasias, Shiny white structures Pigmented: RP, Arborising telangiectasias, White streaks |
Large basaloid lobules with peripheral palisading and dilated blood vessels in the stroma. In pigmented BCC: Colonisation of tumour’s complexes with melanocytes |
| Squamous cell carcinoma | 2 | focal | RP, ulceration, hairpin vessels, scales | Squamous epithelium, abundant keratinisation, intercellular bridges apparent, with dilated blood vessels |
| Melanoma | 2 | multifocal | RP, crust, polymorphic vessels, blue-white veil | Atypical nests and sheets of mitotically active dermal melanocytes |
| Cystic lymphangioma | 1 | focal | RP, lacunae, white septa | - |
| Glomus tumour | 1 | focal | RP, polymorphic vessels, scales | Branching capillary-sized vessels lined by endothelial cells surrounded by collars of uniform glomus cells forming nests and sheets. and trabeculae in a hyalinised or myxoid stroma |
| Keratoacanthoma | 1 | focal | RP, ulceration, hairpin vessels, crusts | large, well-differentiated squamous tumour with a central keratin filled crater |
| Extra genital scleroatrophic lichen | 1 | multifocal | RP, white areas, telangiectasias | Hyperkeratosis, epidermal atrophy with loss of rete ridges, basal layer vacuolisation, homogenised dermal collagen bundles, focally dilated blood vessels surrounded by a discrete inflammatory infiltrate. |
| Pigmented epithelioid melanocytoma | 1 | multifocal | RP, bluish-grey structureless area, darkly pigmented areas | Wedge-shaped and relatively well-circumscribed proliferation of melanocytes and melanophages, with the majority having overlying epidermal hyperplasia and dark, evenly distributed pigmentation |
| Ewing sarcoma | 1 | multifocal | RP, erythematous background, telangiectasias |
Uniform small round cells. Islands separated by dense fibrous tissue with neuroectodermal differentiation. |
| Dermatofibrosarcoma protuberans | 1 | multifocal | RP, erythematous background, crust |
spindle cells with a storiform to whorled pattern. Tumours infiltrate and expand fibrous septa |
| Cutaneous metastasis of a breast cancer | 1 | multifocal | RP, erythematous background, lacunae, polymorphic vessels | interconnecting cords and nests of tumour cells in the dermis and subcutaneous tissue |
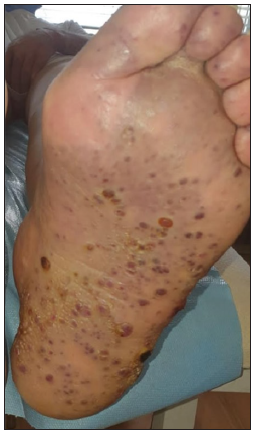
- Clinical image of KS of the foot.
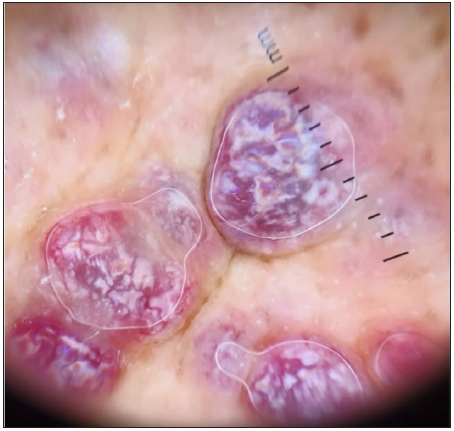
- Dermoscopic image of the KS showing the RP in polarised light (dermoscope dermlite 4,10x).
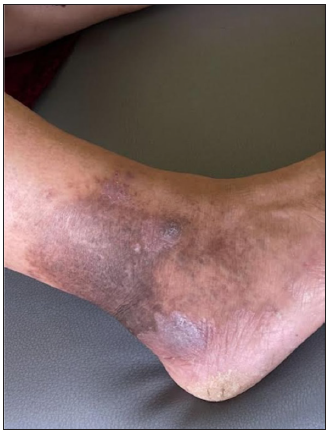
- Clinical image of lichen planus of the lower third of the leg.
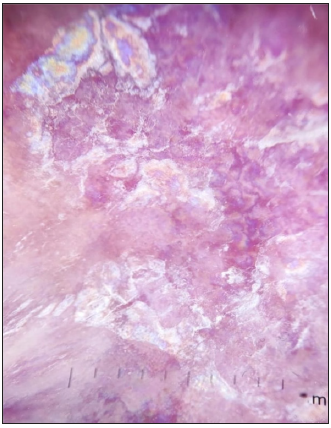
- Dermoscopic image of lichen planus showing the RP in polarised light (dermoscope dermlite 4,10x).
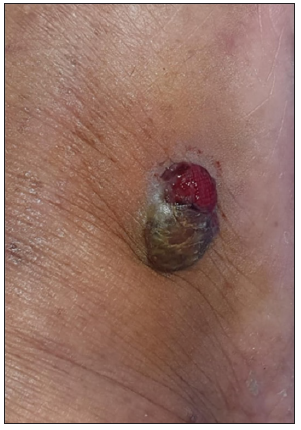
- Clinical image of pyogenic granuloma.
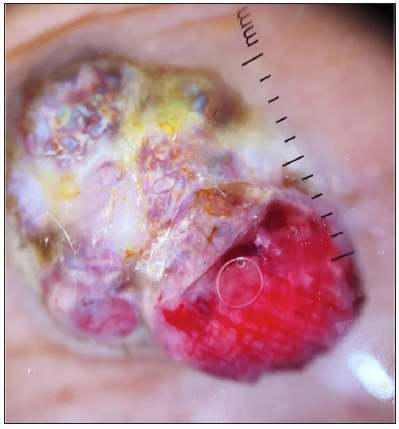
- Dermoscopic image of pyogenic granuloma showing the RP in polarised light (dermoscope dermlite 4,10x).
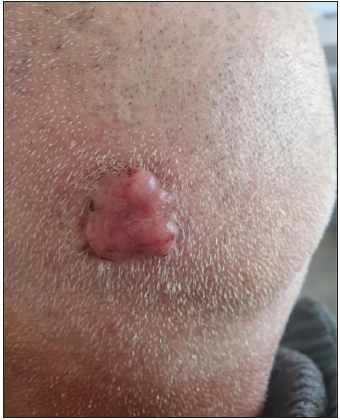
- Clinical image of a non-pigmented BCC of the scalp.
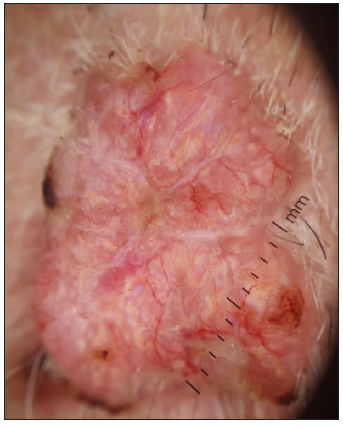
- Dermoscopic image of non pigmented BCC showing the RP in polarised light (dermoscope dermlite 4,10x).
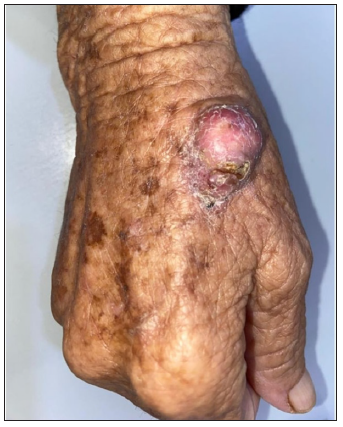
- Clinical image of SCC.
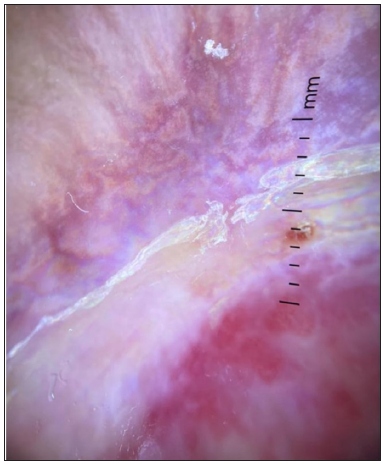
- Dermoscopic image of SCC showing the RP in polarised light (dermoscope dermlite 4,10x).
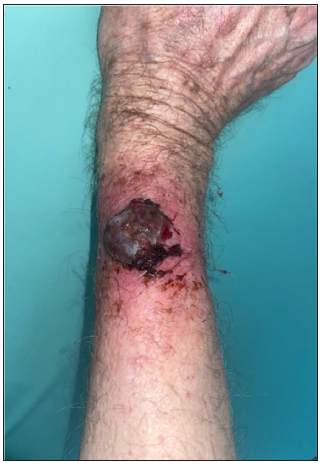
- Clinical image of nodular melanoma.
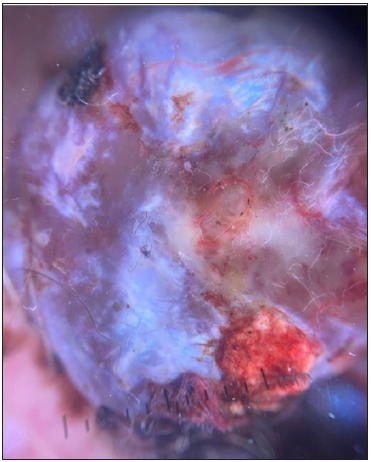
- Dermoscopic image of nodular melanoma showing the RP in polarised light (dermoscope dermlite 4, 10x).
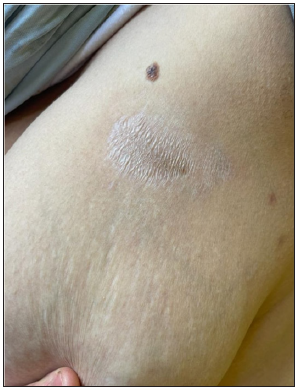
- Clinical image of extra genital scleroatrophic lichen of the trunk.
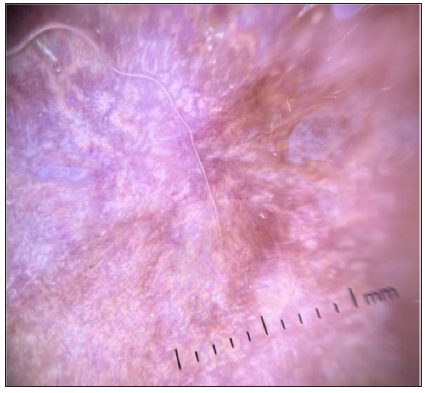
- Dermoscopic image of extra genital scleroatrophic lichen showing the RP in polarised light (dermoscope dermlite 4,10x).
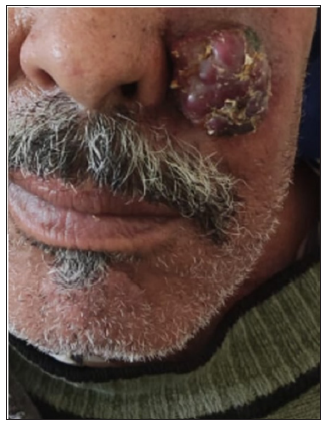
- Clinical image of cutaneous metastasis of breast cancer.
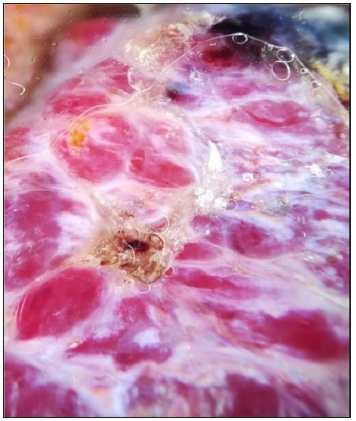
- Dermoscopic image of cutaneous metastasis of breast cancer showing the RP in polarised light (dermoscope dermlite 4, 10x).
The appearance of the RP in dermoscopy is primarily due to the way light interacts with skin structures and lesions.
Cheng et al. explained that the appearance of this pattern is due to the diffraction of the light beam in the dermis of the lesion.1 However, according to Vázquez-López et al., this was partially correct because absorption, diffraction, and interference of polarised light produce colour.2 They emphasised the term dichroism, which means that light in different polarisation states interacts with the superficial and/or deep structural components of the lesion. Each polarisation state will undergo variable absorption and retardation, resulting in a combination of colours induced by absorption and interference.2 This hypothesis explains the appearance of this pattern in vascular and non-vascular lesions.
Satta R et al.,3 suggested that the presence of hyaline globules in KS could contribute to the optical phenomenon producing this pattern in dermoscopy, probably with their birefringence properties. They also indicated that this pattern is observed only in papular- and nodular-type lesions and that it is completely absent in macular and bullous lesions, which is consistent with our results.
In our study, this pattern was found mainly in scars of non-Kaposi’s lesions. This pattern can be observed in both atrophic and hypertrophic scars.4,5
In lichen planus, this pattern has been reported in case reports in dark phototypes.6 The appearance of this pattern has been explained by the rich vascular network, homogeneous diffuse fibrosis, and dense lymphocytic infiltrate of lichenoid dermatosis may contribute to this phenomenon. In this study, three out of five cases with lichen had a dark phototype, so we can also say that the significant presence of pigments favours the appearance of this pattern by its property of dichroism.
In BCC, the pattern was found in the pigmented and non-pigmented form but only in nodular lesions, which is consistent with the results of Suppa et al.7
Cases of angiokeratoma, pyogenic granuloma, melanoma, SCC, keratoacanthoma, dermatofibroma, and glomus tumour have been previously described as having an RP.2,4,5 To our knowledge, no RP has been previously described for cystic lymphangioma, Extra genital scleroatrophic lichen, pigmented epithelioid melanocytoma, Ewing sarcoma, and cutaneous metastasis of breast cancer. We observed a clear predominance of this pattern in richly vascularised lesions since they interact more with polarised light. Moreover, the presence of this pattern in benign and malignant tumours does not make it a dermoscopic indicator of malignancy.
Thus, the RP is an optical phenomenon that has no particular diagnostic significance. It can be found in Kaposi’s and non-Kaposi, vascular and non-vascular lesions. The RP alone cannot guide a diagnosis, but a global analysis of the lesion, particular the clinical presentation, other associated dermoscopic signs, and finally, the histology in some cases, is important to make a final diagnosis.
Ethical approval
This study is an analysis of anonymized dermoscopic images, without any interventions on patients, including management. So the institutional review approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent..
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Rainbow pattern in Kaposi’s sarcoma under polarized dermoscopy: A dermoscopic pathological study. Br J Dermatol. 2009;160:801-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic rainbow pattern in non-kaposi sarcoma lesions. Br J Dermatol. 2009;161:474-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic rainbow pattern in kaposi’s sarcoma lesions: Our experience. Arch Dermatol. 2012;148:1207.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic rainbow pattern: A strong clue to malignancy or just a light show? North Clin Istanb. 2020;7:494-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The rainbow pattern in dermoscopy: A zoom on nonkaposi sarcoma skin diseases. Biomed J. 2018;41:209-10.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopic rainbow pattern in lichen planus pigmentosus inversus in a middle-aged African American man. JAAD Case Rep. 2023;35:118-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dermoscopic variability of basal cell carcinoma according to clinical type and anatomic location. J Eur Acad Dermatol Venereol. 2015;29:1732-41.
- [CrossRef] [PubMed] [Google Scholar]





