Translate this page into:
The utility of etanercept in chronic stable plaque psoriasis: Results from an open-label, prospective, single arm study
Correspondence Address:
Rashmi Singh
Department of Dermatology, Government Stanley Medical College and Hospital, Royapuram, Chennai - 600 001, Tamil Nadu
India
| How to cite this article: Venkatesan A, Saradha K P, Kumar V S, Kumar MS, Begum C Z, Singh R. The utility of etanercept in chronic stable plaque psoriasis: Results from an open-label, prospective, single arm study. Indian J Dermatol Venereol Leprol 2017;83:113-116 |
Sir,
The introduction of biologicals has greatly revolutionized the treatment options for psoriasis, with the advantages of good efficacy and a reasonable safety profile.
The recombinant tumor necrosis factor antagonist, etanercept is approved for use in India as monotherapy in adults and children (>6 years) with moderate to severe psoriasis. Extensive studies from around the world, in adults with moderate to severe plaque psoriasis, demonstrate its long-term benefits. However, Indian data is limited.
We conducted an open-label, non-randomized, single-arm, prospective, observational study in patients with chronic plaque-type psoriasis in order to observe the safety, efficacy and tolerability of subcutaneously injected etanercept (50 mg/week), at the Department of Dermatology, Government Stanley Medical College, Chennai. Data was collected between June 2012 and June 2013. The study was approved by the institutional ethics committee and informed consent was obtained from all patients.
A detailed medical history and physical examination findings were recorded at the initial visit. Twenty patients with chronic stable plaque type psoriasis satisfying the inclusion criteria [Table - 1] were enrolled in this study. Baseline investigations were undertaken [Table - 2].

Patients were hospitalized and administered weekly subcutaneous injections of etanercept 50 mg for 12 weeks and an additional 12-week safety follow-up was conducted. Assessment of the safety profile of etanercept was done using the National Cancer Institute's Common Toxicity Criteria, version 2.0, 1999.
Efficacy was assessed by monitoring the psoriasis area severity index score on a weekly basis throughout the duration of the study. Clinical improvement was graded as 'good' on achieving PASI 50 (50% or more reduction in psoriasis area severity index) and 'excellent' on achieving PASI 75 (75% or more reduction in psoriasis area severity index).
There were 10 men and 10 women with a mean age of 39.5 years and a mean duration of psoriasis of 9 years. The mean psoriasis area severity index score at baseline was 22.8. Eighteen patients had received prior systemic therapy or phototherapy.
At week 4, only 1 (5%) patient achieved PASI 25 (25% reduction in psoriasis area severity index) while the rest had less than 25% reduction in psoriasis area severity index. At week 8, 8 (40%) patients achieved PASI 50 (50% reduction in psoriasis area severity index), 9 (45%) patients achieved PASI 25 and 3 (15%) had patients less than 25% reduction.
At week 12, 13 (65%) patients had achieved PASI 75 (primary endpoint), 5 (25%) patient had achieved PASI 50, 1 (5%) patient had achieved PASI 25 and 1 patient had less than 25% reduction in psoriasis area severity index. Similarly, the mean psoriasis area severity index score was observed to decrease from 22.8 at baseline to 20.8 at the end of week 4, 12.8 at week 8 and 7.2 (68.4%) at the end of week 12 [Figure - 1] and [Figure - 2]a,[Figure - 2]b,[Figure - 2]c.
 |
| Figure 1: Decline in mean psoriasis area severity index score over 12 weeks |
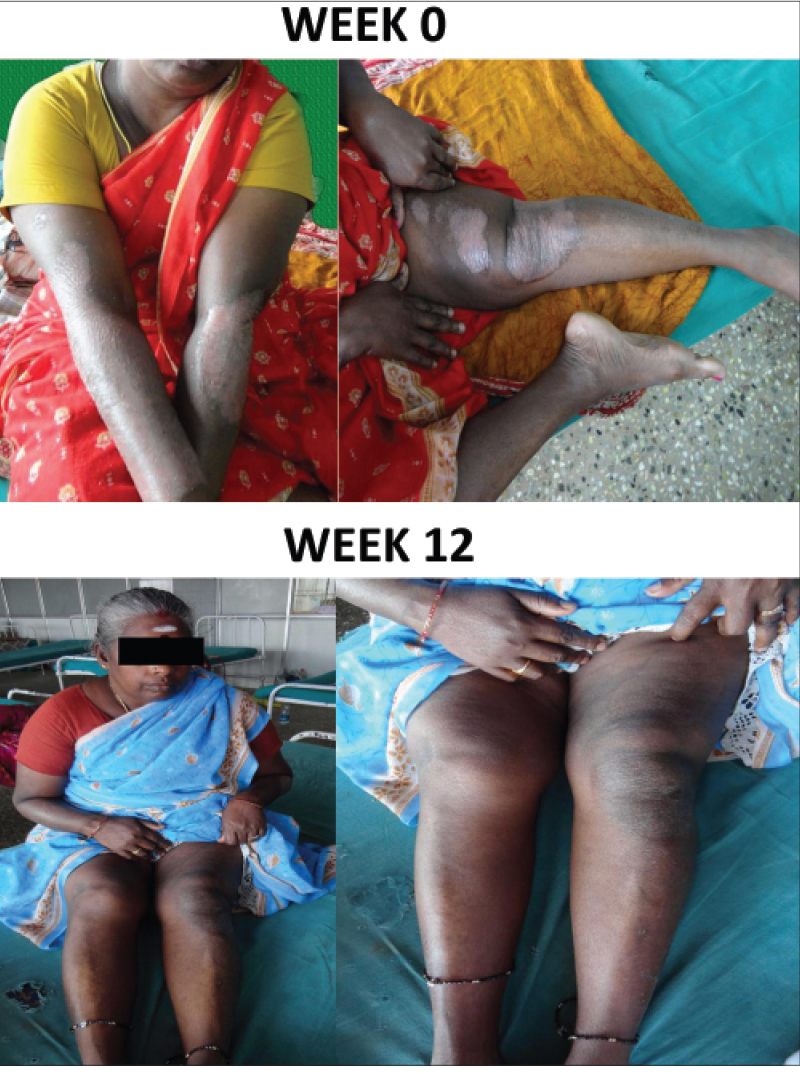 |
| Figure 2a: Patient Response A: Week 0 and 12 |
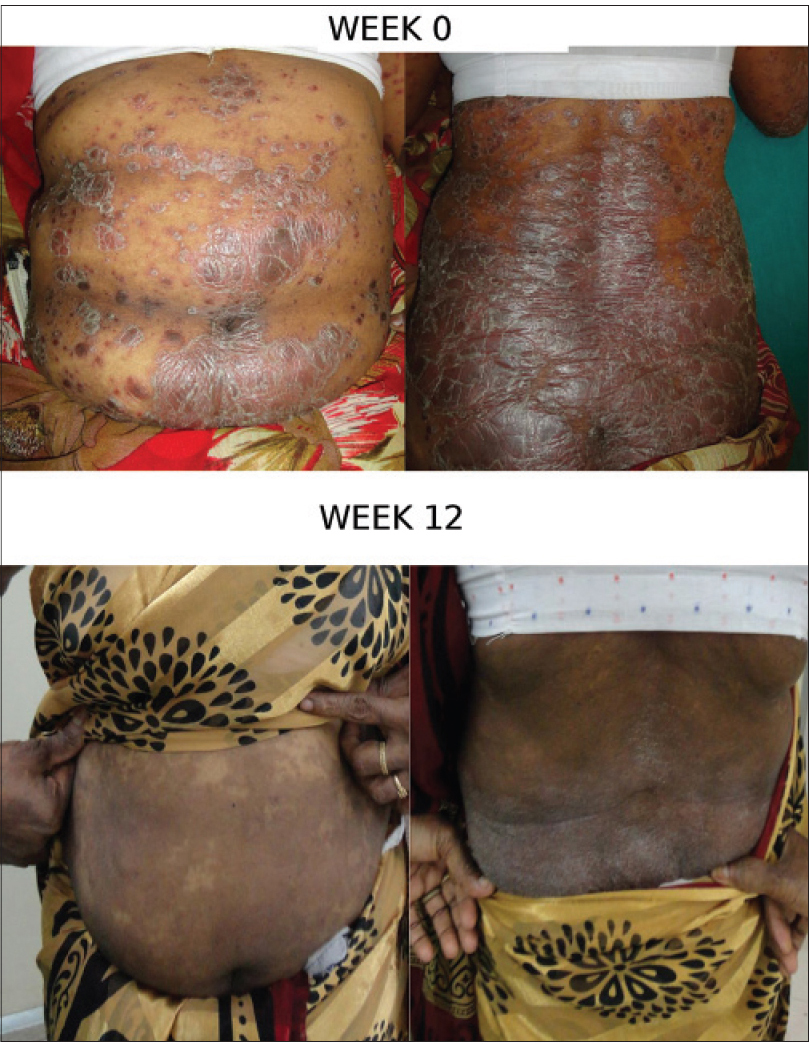 |
| Figure 2b: Patient Response B: Week 0 and 12 |
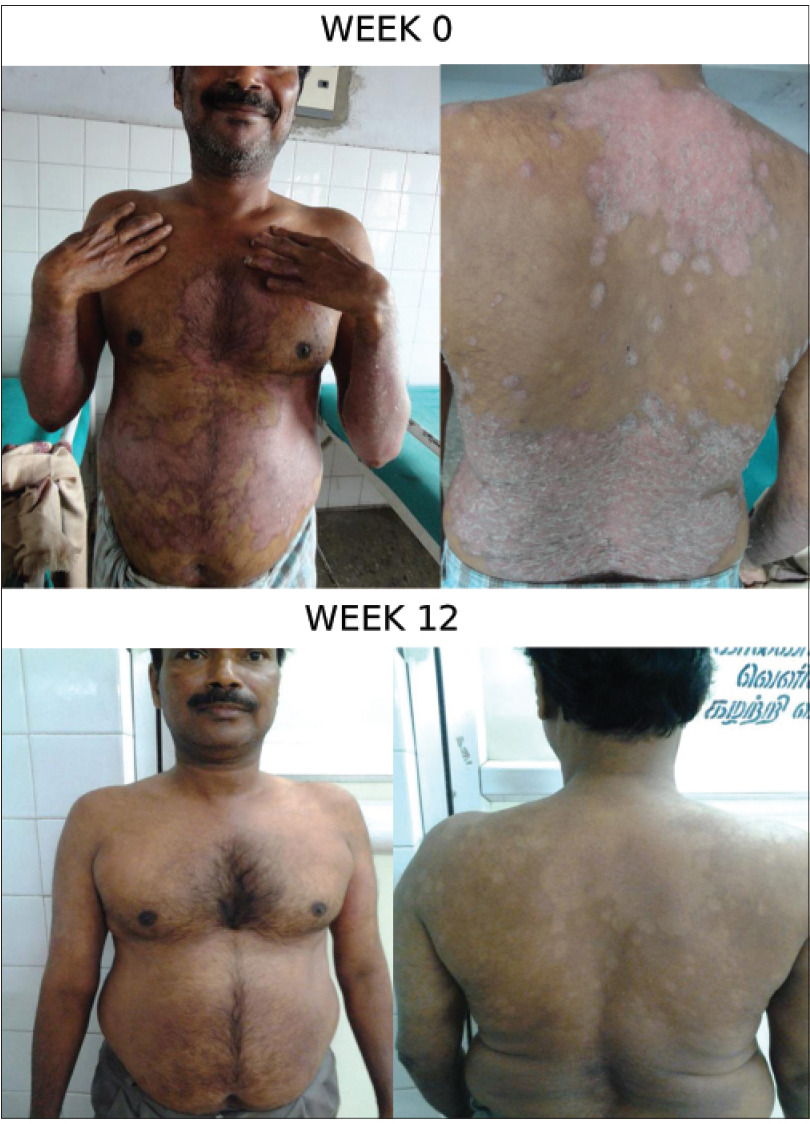 |
| Figure 2c: Patient Response C: Week 0 and 12 |
No hematological, cutaneous or systemic adverse events were observed in this study. No specific adverse events leading to withdrawal occurred during this study after 12 weeks. In general, etanercept was well tolerated.
The mean duration of remission was 3 months (standard deviation = 2.35).
Our results correlated with the study conducted by Sterry et al. who reported that 62% of patients treated with injection etanercept 50 mg/week achieved PASI 75 (75% reduction in psoriasis area severity index) at week 12.[1] On the other hand, studies conducted by Leonardi et al. and Papp et al. showed that only around one-third of the patients receiving injection etanercept 50 mg/week achieved PASI 75 at week 12.[2],[3] The mean time to relapse was 84 days which concurred with our results.[3] Another study by Papp et al. showed that etanercept was clinically beneficial to patients with chronic plaque psoriasis, with no apparent decrease in efficacy after dose reduction from 50 to 25 mg twice weekly.[4]
Even in the era of biologics, methotrexate remains the first-line drug in the treatment of moderate to severe psoriasis and psoriatic arthritis. Unfortunately, its dose-dependent risk of hepatotoxicity remains high.
Mazzotta et al. observed that patients who have not taken any biological therapy earlier have a better response to treatment with etanercept.[5] None of our patients had been exposed to biologicals previously. A study conducted in Italy, by calculating quality adjusted life years, inferred that in patients with psoriasis area severity index >20, etanercept is a cost-effective treatment.
Numerous published psoriasis trials have shown that etanercept is well tolerated. In placebo-controlled trials, adverse events have been typically reported to be mild to moderate in intensity, and occuring with similar frequency in both groups. Psoriasis clinical trials have revealed no evidence of increased risk of opportunistic infections, tuberculosis, or skin cancers during upto 60 weeks of etanercept treatment (Gottlieb et al., unpublished data, 2004).
The results from this study indicate that etanercept provided therapeutic improvement and was well tolerated in the treatment of stable chronic plaque psoriasis in our patients. Given the scarcity of available data, these findings from a small case series in an Indian tertiary level teaching hospital may be of value.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ 2010;340:c147.
[Google Scholar]
|
| 2. |
Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349:2014-22.
[Google Scholar]
|
| 3. |
Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J Am Acad Dermatol 2012;66:e33-45.
[Google Scholar]
|
| 4. |
Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. Aglobal phase III randomized controlled trial of etanercept in psoriasis: Safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304-12.
[Google Scholar]
|
| 5. |
Mazzotta A, Esposito M, Costanzo A, Chimenti S. Efficacy and safety of etanercept in psoriasis after switching from other treatments: An observational study. Am J Clin Dermatol 2009;10:319-24.
[Google Scholar]
|
Fulltext Views
3,739
PDF downloads
2,370





