Translate this page into:
Tinea capitis in the pediatric population: A study from North India
2 Department of Microbiology, Chacha Nehru Bal Chikitsalaya, Geeta Colony, New Delhi, India
Correspondence Address:
Chander Grover
Department of Dermatology, University College of Medical Sciences and Associated Guru Teg Bahadur Hospital, Dilshad Garden, Delhi
India
| How to cite this article: Grover C, Arora P, Manchanda V. Tinea capitis in the pediatric population: A study from North India. Indian J Dermatol Venereol Leprol 2010;76:527-532 |
Abstract
Background: Tinea capitis (TC) is a common superficial fungal infection seen predominantly in children. The etiological factors vary from one region to the other. The clinical and microbiological characteristics of the same were studied in patients up to the age of 12 years seen at a pediatric superspeciality hospital in New Delhi, India. Aims: To delineate the various patterns of TC observed in North India and to assess for any correlation between the clinical, microscopic and microbiologic findings in the patients seen. Also, to identify the common fungal species responsible for producing TC in North India. Methods: Clinical morphology and KOH findings were studied in 214 patients with the suspected diagnosis of TC. Fungal culture were also performed for all the cases. An attempt was made to evaluate any correlation among the clinical, microscopic and etiological findings. The epidemiological factors associated with the disease were also assessed. Results: TC was found to be most common in the 8-10-year age group, with noninflmmatory TC being the more common type (56.5%). A mixed morphological pattern was recorded in 10% of the cases. Microscopic examination revealed an endothrix pattern of hair invasion to be more common (41.5% cases). Again, 8.8% of the cases showed foci of both endothrix and ectothrix pattern of invasion simultaneously. Trichophyton violaceum was the most common fungal species isolated. Conclusions: In the present study, clinical morphology or KOH findings were not found to be clearly or exclusively predictive of the species involved. There was a fair degree of overlap in the clinical or microscopic patterns produced by the fungal species. Mixed patterns were observed both on clinical examination as well as on KOH examination. However, none of the specimens grew more than one fungal species.Introduction
Tinea capitis (TC) is a fungal infection of the scalp, hair follicles and hair shafts, especially common in the pediatric population and under tropical conditions. [1] The highest incidence is seen in children 3-7 years of age. [2] The presence of symptoms like hyperkeratosis of scalp, seborrhea-like symptoms, excoriation secondary to pruritus, alopecia, broken hair or "black dot" appearance, cervical lymphadenopathy, pustules, or indurated or boggy plaques in a child should alert the dermatologist toward the possibility of TC. [3]
TC is caused by various dermatophytes. The prevalence of various causative fungi varies according to the geographical area being studied. TC can present as noninflammatory or inflammatory morphological variants. [4] An early diagnosis is important to prevent transmission between children, especially siblings, and also to avoid possible scarring and permanent hair loss. The presence of tinea corporis or an id reaction in a child should also prompt a search for TC. [5]
As is true for most infectious diseases, the epidemiology of TC is in a constant state of flux, and varies considerably with respect to geography and specific population groups. [6] This fact prompted the present study, aimed at evaluating the clinical and etiologic profile of TC in a pediatric super-specialty hospital in the city of Delhi, India.
Methods
A prospective, cross-sectional study was carried out by the Departments of Dermatology and Microbiology at a pediatric super-specialty hospital in New Delhi. The Institutional Review Board approved the study. All children up to 12 years of age, presenting to the Dermatology Outpatient Department between April 2006 and December 2008 with a suspected diagnosis of TC were enrolled for the study. These included children presenting with patchy hair loss and easy pluckability of hair, with or without any associated inflammatory changes. Patients on any oral or topical antifungal therapy for the past 6 weeks were excluded from the study.
A detailed history was taken regarding the duration and pattern of hair loss. Demographic and socioeconomic data of the patients were recorded. Factors predisposing toward the spread of TC were analyzed. This included an assessment of the living conditions, history of any other family members affected and the hair care practices being followed. The patients were specifically asked about pets or any other prolonged contact with animals.
Examination of the whole scalp was carried out to assess the type and extent of hair loss. Patients were classified according to the morphological types of TC as noninflammatory (black dot [BD], gray patch [GP], seborrheic dermatitis type), inflammatory (pustular, kerion or favus) or mixed infection (any combination of the above). The patients were thoroughly examined to assess for any evidence of tinea corporis, nail involvement, id reaction or lymphadenopathy. Siblings of affected children as well as close family contacts were also examined to assess for any hair loss.
The patients were asked to come after head wash so as to remove any oil from the scalp. For all the patients, skin scrapings and hair fragments were collected from the affected areas in an aseptic manner. The material collected on a slide was immersed in potassium hydroxide (10% KOH) to prepare smears for microscopic examination. The slides were assessed under a low-power microscope to look for fungal arthrospores or any hyphae. If the spores were located on the surface of the hair shaft, without causing any distortion of hair architecture, the infection was classified as ectothrix. If the spores were seen inside the shaft and were destroying the hair fragment architecture, the infection was classified as endothrix. Presence of both the patterns in the same specimen was also recorded.
The samples were also inoculated on Sabouraud′s Dextrose Agar; with and without antibiotics (chloramphenicol, gentamicin and cycloheximide). This was done to identify the causative species involved. The clinical, microbiological and etiologic data were collected and correlated.
Results
A total of 214 cases with suspected TC seen over a 2.5-year period were enrolled for the study. Children up to the age of 12 years were included in the study, and the youngest of these was 6 months of age. The mean age of the affected children was 6.8 3.05 years. A majority of the children (25.2%) belonged to the 8-10-year age group [Graph 1]-[SUPPORTING:1]. A slight female preponderance was seen, with females comprising 51.4% of the patients.
Approximately 74% of the children belonged to the lower middle and 19% belonged to the lower income groups. There were 15 sets of siblings among these patients, with each set having two to three children. A history of sharing of combs and hair accessories was elicited in 62% of the patients. Daily application of oil was a rampant practice present in 78% of the children examined. Only 14% gave a history of pets at home or prolonged contact with animals. Pets in all the cases were reported to have no skin complaints.
Among the various clinical variants seen, noninflammatory TC was more common (56.5%) than inflammatory TC (32.7%) [Table - 1]. A mixed morphological pattern was seen in 10% of the cases [Figure - 1]. No cases of favus were seen. Among the noninflammatory cases, black dot tinea capitis (BDTC) was more common than the other types. Among the inflammatory cases, pustular variant was more common than the kerion.

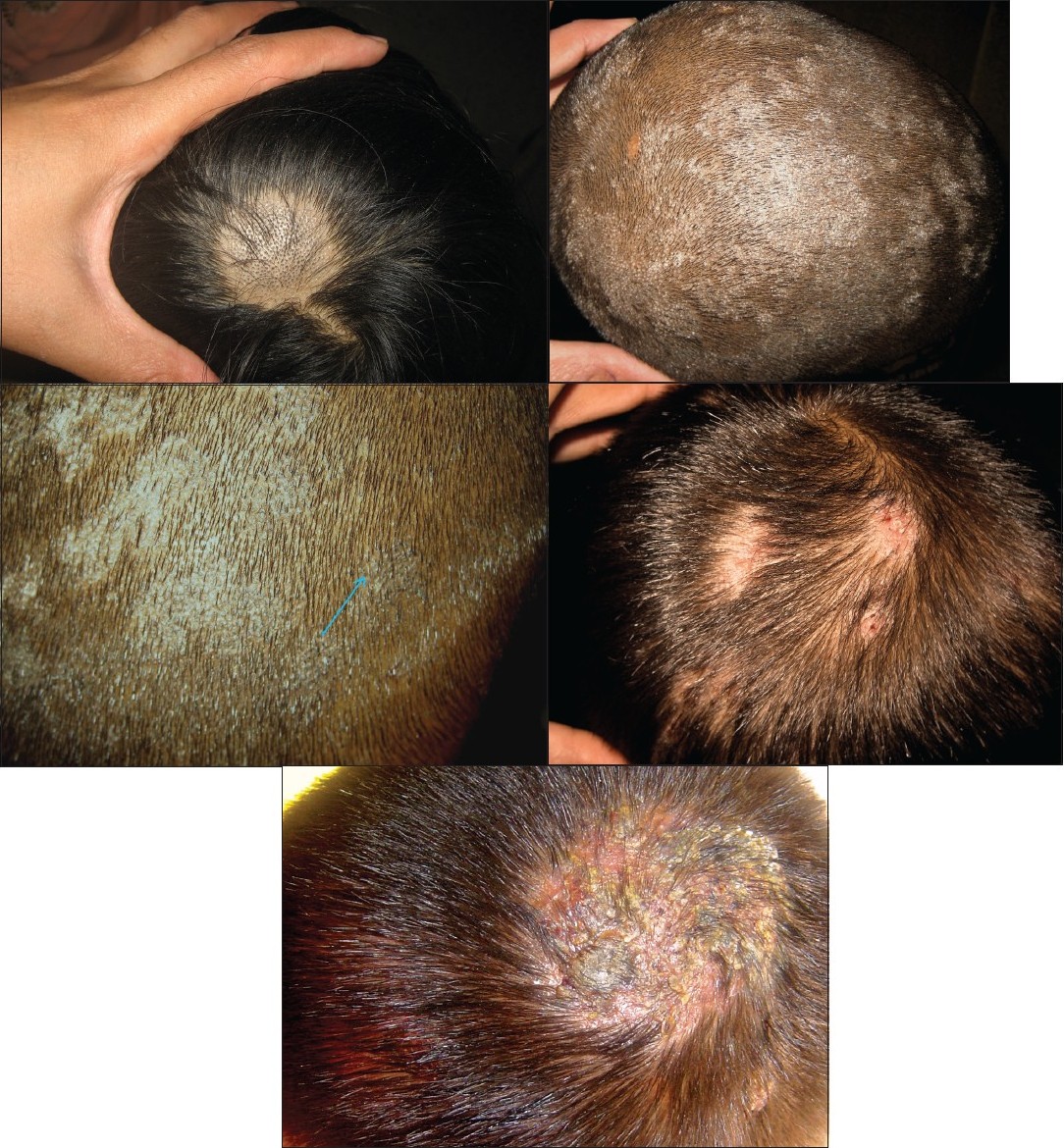 |
| Figure 1 :(a) Black dot tinea capitis (BDTC) Figure 1b: Gray patch tinea capitis (GPTC) Figure 1c: Mixed morphology: a child with GPTC showing distinct focus with BDTC (shown with an arrow). There is absence of scaling in the area with BDTC Figure 1d: A case of pustular tinea capitis showing discrete foci of involvement Figure 1e: A child with kerion presenting as a boggy swelling over the scalp |
Hair roots were examined under 10% KOH in all the cases. The findings are summarized in Graph 2-[SUPPORTING:2]. Fungal spores invading hair shafts could be seen in 82.3% (176) of the cases. An endothrix pattern of spore distribution was more common (41.5%) than an ectothrix pattern (31.7%) [Figure - 2]. Interestingly, 19 cases (8.8%) showed spores in both the patterns simultaneously on initial examination. No fungal elements could be identified in 38 cases (17.7%).
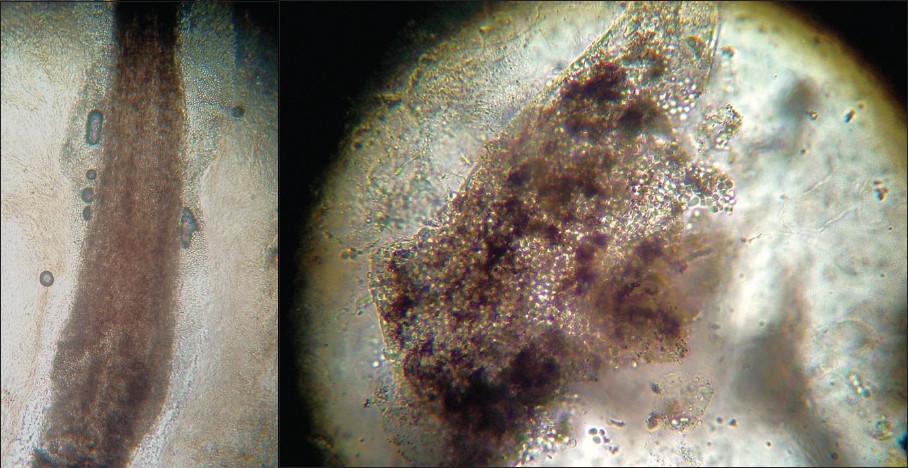 |
| Figure 2 :(a) Microscopic patterns of tinea capitis: (a) Ectothrix – a hair shaft surrounded by fungal spores lying in chains over the surface of hair Figure 2b: Endothrix – fungal spores invading the hair shaft. The hair shaft is broken and has an altered morphology |
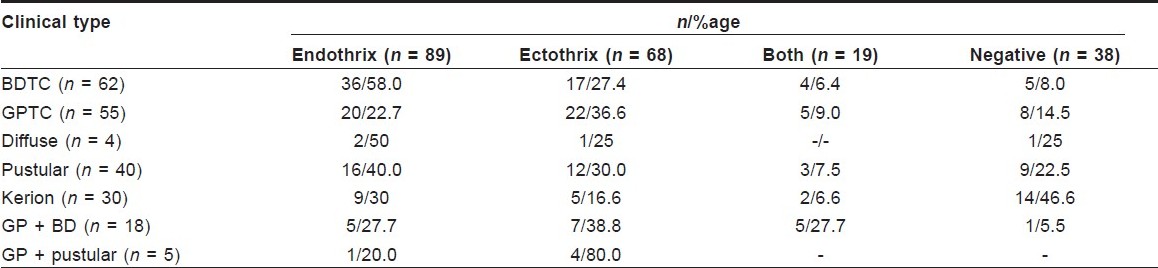
An attempt was made to correlate the clinical and microscopic types of TC [Table - 2]. It was seen that although the endothrix pattern was more common for BDTC, the ectothrix pattern is also seen in a large number of cases. Similarly, ectothrix invasion is more common for gray patch tinea capitis (GPTC), although endothrix cases can also be seen. Significantly, almost half the cases with kerion did not reveal any fungal elements on microscopic examination. Also, most of the cases with a mixed pattern of invasion on KOH belonged to the GP + BD morphology.
Culture specimens from all the cases were examined. No growth at the end of 6 weeks was recorded in 27.1% of the cases (58 cases). Of those showing growth of fungal elements, Trichophyton violaceum was the most common isolate in 88.6% (138 cases). This was followed by T. rubrum (four cases), T. tonsurans (three cases), Microsporum audouinii (three cases), T. terrestrae (two cases) and contaminant growth (six cases).
A correlation of fungal species isolated with the clinical type of TC was drawn out [Table - 3]. It can be seen that T. violaceum was responsible for BDTC in most of the cases. T. terrestrae and M. audouinii were responsible for a GPTC pattern. T. tonsurans was isolated from cases of kerion and T. rubrum from cases with pustular TC.

Discussion
TC is a common fungal infection, particularly among children in urban regions. [7] More often than not, it presents with mild scaling and little hair loss, which is reversible. However, in a few cases, it may be characterized by intense inflammation and subsequent cicatricial alopecia, which causes permanent cosmetic disfigurement. Also, the infection is highly contagious and, hence, needs to be recognized and treated early to prevent transmission to siblings and costudents.
The 214 children studied by us were from an urban set-up, and mostly belonged to the age group of 8-10 years. This is unlike previous reports that have shown a higher incidence in the 4-7-years age group. [6],[7]
This could be because the 8-10-years age group included school going children who were at a higher risk of transmission of the disease because of close contact with each other. [6] Beyond this age group, the incidence declines because of the onset of puberty and seborrhea.
Various conflicting views exist regarding the sexual predominance of TC. Some authorities believe that TC may be common in boys due to shorter hair, allowing easy access for circulating spores, [8] while others believe that it may be more common in girls due to tight hair braiding. [6] An almost equal number of males and females were affected in our series, similar to previous study by Singal et al. from North India. [9]
As seen in earlier reports, noninflammatory types of TC were more common (51%) than inflammatory variants (32%) in our series as well. [9],[10],[11],[12],[13] Among the noninflammatory cases, BD and GP morphology was found at an almost equal level of frequency. Earlier reports have variably shown BD [11],[12] or GP [10],[13] to be the most common morphological types. Diffuse scaling (similar to seborrheic dermatitis) was the least common type of TC in our series, unlike the study by Singal et al.[9]
Inflammatory TC accounted for 32% of the cases, which was similar to earlier reports. [13],[14] Mostly, these cases showed widespread pustules with patches of cicatricial alopecia (pustular TC). Kerion accounted for 13.5% of the cases. No cases of favus were seen.
An interesting finding in our study was the presence of mixed morphology in 10% of the cases. These were of two types. Mostly, it presented as discrete areas in scalp showing either BDTC or GPTC morphology, present in the same patient. The other type included children presenting initially with GPTC morphology, but who went on to develop pustules in a widespread distribution over the scalp within 1 week of starting treatment. Although previous studies have described isolation of multiple dermatophyte species from a single patient, [15] the coexistence of more than one morphological type of TC in a single child has only rarely been reported. [16]
Examination of KOH-stained smears microscopically revealed different patterns of distribution of spores in the hair shaft. A positive result was seen in 82.3% of the cases. This is in accordance with earlier reports where positive results had been seen in 89.6% of the cases. [17] Similar to previous reports, endothrix presentation (41.5%) was more common than ectothrix (31.7%) in our series as well. [9] Interestingly again, 7.3% of the cases showed broken hair stumps with spores in an ectothrix as well as endothrix pattern. These cases had GP + BD or pustular morphology [Table - 2]. Of the cases showing no fungal elements on KOH, the majority belonged to inflammatory types of TC (kerion or pustular).
According to the pathogenesis of TC, endothrix distribution of spores produces BDTC morphologically and the ectothrix pattern clinically looks like GPTC. It can be seen from Table 2 that most of the cases of BDTC were endothrix and of GPTC were ectothrix. However, these categories were not mutually exclusive. This could be because of more than one species being present in a patient (mixed infection) or a species producing more than one pattern of hair invasion and, hence, morphology. Previous studies have shown that clinical presentation is not correctly indicative of the type of fungus or vice versa, as it also depends on other unknown factors. [9],[13]
Fungal culture gives valuable data with respect to the species involved in producing the disease. The culture yielded positive result in 73% of the cases. Earlier reports have shown a positive isolation rate of 56-92%. [10],[15] The predominant species seen by us was T. violaceum (responsible in almost 88.6% of all the culture-positive cases). This could be because our catchment area was a densely populated area, allowing close contact between children. Also, a large number of cases were siblings and could have harbored the same species (15 set of siblings). T. violaceum is the most common fungus in India, [11] Nepal [10] and Pakistan. [14] In the literature, it has also been reported to be the most common isolate from South Africa and the UK. [16] Other organisms were seen to a very low extent. Sidat et al. had reported the isolation of multiple isolates from a single specimen. [15] Interestingly, none of our samples showed growth of more than one fungal species, as may have been expected from morphology or KOH examination. We failed to isolate any cases with T. schoenlenii, unlike previous studies from similar geographical areas. [9],[17]
Our study threw up interesting findings. BDTC was the most common type of TC and T. violaceum was the most common species isolated. KOH examination and culture were useful diagnostic methods. However, the clinical morphology or KOH findings were not found to be clearly predictive of the species involved. Mixed patterns were observed both on clinical examination as well as on KOH examination. However, none of the specimens grew more than one fungal species. Studies involving a larger number of patients and more sensitive fungal isolation may help generate more information on this issue.
| 1. |
Ghannoum M, Isham N, Hajjeh R, Cano M, Al- Hasawi F, Yearick D, et al. Tinea capitis in Cleveland: Survey of elementary school students. J Am Acad Dermatol 2003;48:189-93.
[Google Scholar]
|
| 2. |
Elewski BE. Tinea capitis: A current perspective. J Am Acad Dermatol 2000;42:1-20.
[Google Scholar]
|
| 3. |
Alvarez MS, Silverberg NB. Tinea capitis. Cutis 2006;78:189-96.
[Google Scholar]
|
| 4. |
Hay RJ, Moore MK. Mycology. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 7 th ed. Massachusetts: Blackwell Publishing; 2004. p. 31.1-31.101.
th ed. Massachusetts: Blackwell Publishing; 2004. p. 31.1-31.101. '>[Google Scholar]
|
| 5. |
Rasmussen JE, Ahmed AR. Trichophytin reactions in children with tinea capitis. Arch Dermatol 1978;114:371-2.
[Google Scholar]
|
| 6. |
Chen BK, Friedlander SF. Tinea capitis update: a continuing conflict with an old adversary. Curr Opin Pediatr 2001;13:331-5.
[Google Scholar]
|
| 7. |
Trovato MJ, Schwartz AR, Janninger CK. Tinea capitis: current concepts in clinical practice. Cutis 2006;77:93-9.
[Google Scholar]
|
| 8. |
Friedlander SF, Rueda M, Chen BK, Caceros-Rios HW. Fungal, protozoal and helminthic infections. In: Schachner LA, Hansen RC, editors. Pediatric Dermatology. 3 rd ed. Mosby; 2003: p 1093-140.
[Google Scholar]
|
| 9. |
Singal A, Rawat S, Bhattacharya S, Mohanty S, Baruah MC. Clinico- mycological profile of tinea capitis in North India and response to griseofulvin. J Dermatol 2001;28:22-6.
[Google Scholar]
|
| 10. |
Jha BN, Garg VK, Agrawal S, Khanal B, Agarwalla A. Tinea capitis in Eastern Nepal. Int J Dermatol 2006;45:100-2.
[Google Scholar]
|
| 11. |
Kalla G, Begra B, Solanki A, Goyal A, Batra A. Clinico mycological study of Tinea capitis in Desert District of Rajasthan. Ind J Dermatol Venereol Leprol 1995;61:342-5.
[Google Scholar]
|
| 12. |
Hussain I, Aman S, Haroon TS, Jahangir M, Nagi AH. Tinea capitis in Lahore, Pakistan. Int J Dermatol 1994;33:255-7.
[Google Scholar]
|
| 13. |
Kumar AG, Lakshmi N. Tinea capitis in Tirupati. Ind J Pathol Microbiol 1990;33:360-3.
[Google Scholar]
|
| 14. |
Jahangir M, Hussain I, Khurshid K, Haroon TS. A clinico-etiologic correlation in Tinea capitis. Int J Dermatol 1999;38:275-8.
[Google Scholar]
|
| 15. |
Sidat MM, Correia D, Buene TP. Tinea capitis among children at one suburban primary school in the city of Maputo, Mozambique. Revista da Sociedade de Medicina Tropical 2000;40:4/3-4-5.
[Google Scholar]
|
| 16. |
Mills CM, Philpot CM. Tinea capitis in south Wales-observations in change of causative fungi. Clin Exp Dermatol 1994;19:473-5.
[Google Scholar]
|
| 17. |
Jain N, Sharma M, Saxena VN. Clinico-mycological profile of dermatophytosis in Jaipur, Rajasthan. Indian J Dermatol Venereol Leprol 2008;74:274-5.
[Google Scholar]
|
Fulltext Views
10,317
PDF downloads
4,047





