Translate this page into:
Topical atorvastatin in the management of porokeratosis
Corresponding author: Dr. Rahul Mahajan, Department of Dermatology, PGIMER, Chandigarh, India. drrahulpgi@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Janaani P, Chatterjee D, Mahajan R. Topical atorvastatin in the management of porokeratosis. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_575_2024
Dear Editor,
Porokeratosis is a heterogeneous group of disorders of keratinisation with disseminated superficial actinic porokeratosis (DSAP) being the most prevalent form.1 Lesions appear as asymptomatic or itchy brown papules or plaques on photo-exposed regions with an atrophic or hypopigmented centre and a border looking like a raised railroad track. Though the exact pathogenesis is not known, interference of mevalonate kinase pathway in cholesterol synthesis has been shown to play some role. There are multiple therapeutic options for porokeratosis like cryotherapy, photodynamic therapy, LASERs, topical imiquimod, topical 5-fluorouracil, oral retinoids, topical steroids and vitamin D analogues, but these are not much effective and are costly. Recent research has demonstrated that treating porokeratosis with topical lovastatin and cholesterol can be effective.2,3
A 66-year-old male presented with multiple itchy brownish lesions over face, trunk and extremities of one year duration. On examination, multiple brownish papules and annular plaques were seen over the above-mentioned areas with central atrophy and thin raised thread-like border the size of 0.5–1 cm and minimal scaling. Figure 1a and 1b demonstrates baseline clinical images of annular plaques over trunk and forearms respectively. Histopathological examination of skin biopsy from the border of one of the lesions revealed focal invagination of epidermis with a column of parakeratosis, hypogranulosis and apoptotic keratinocytes (coronoid lamella). Figure 2 illustrates histopathological changes seen in this patient. With the diagnosis of dissemination superficial porokeratosis (DSP), the patient was started on low-dose acitretin 10 mg daily but with minimal improvement even after one year of therapy. Thereafter, the patient was counselled regarding the possible usefulness of topical statins in his skin disease. Since topical lovastatin is not commercially available, we decided to compound topical 2% atorvastatin cream in our pharmacy. It was prepared by mixing crushed tablets of atorvastatin 20 mg (10 tablets) in 10 gms of white paraffin wax, making it 2% atorvastatin cream. We advised the patient to apply half a fingertip unit of cream to bigger lesions (approximately 2 cm), which is changed appropriately to lesional size. After one month of treatment with the combination of oral acitretin with 2% atorvastatin cream, the patient reported 40% improvement in itching; hence oral acitretin was discontinued. At the end of four months of therapy, there was complete resolution of pruritis and few lesions started to resolve. Figure 3a and 3b illustrates flattening of all lesions and 20% resolution of old lesions after 6 months of treatment. The patient reported an overall improvement in the quality of life. No adverse effects were observed.
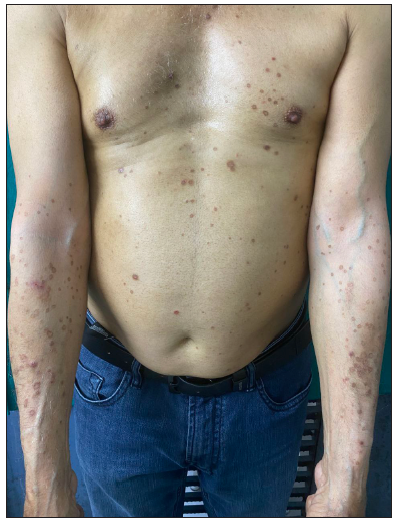
- At baseline, there are multiple brownish papules and annular plaques over the trunk and forearms.
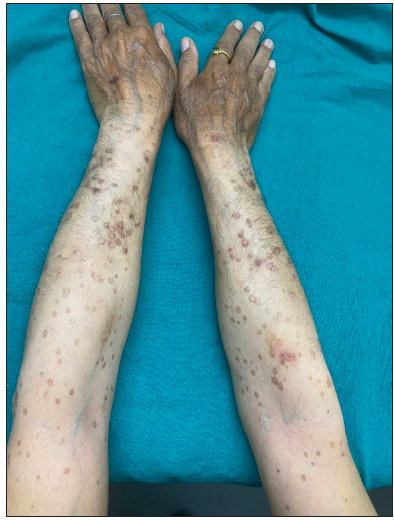
- Lesions with central atrophy and thin raised thread-like border.
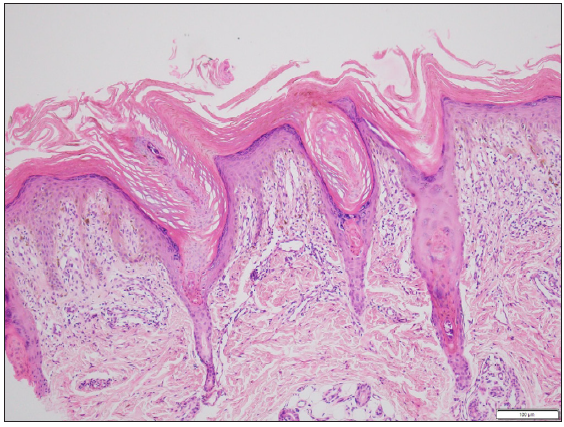
- Histopathological examination of skin biopsy from the border of one of the lesions revealed focal invagination of epidermis with a column of parakeratosis, hypogranulosis and apoptotic keratinocytes (coronoid lamella) (Haematoxylin and eosin stain, 100x).
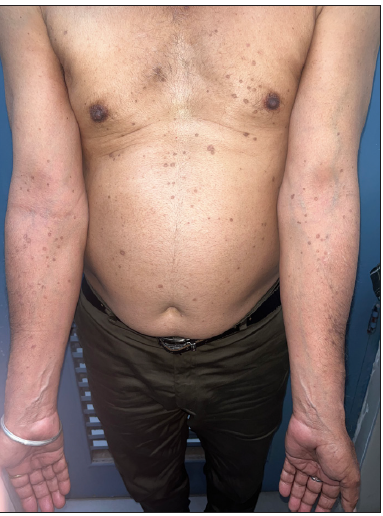
- Decrease in the number of lesions over the trunk and upper limbs 6-months post treatment.
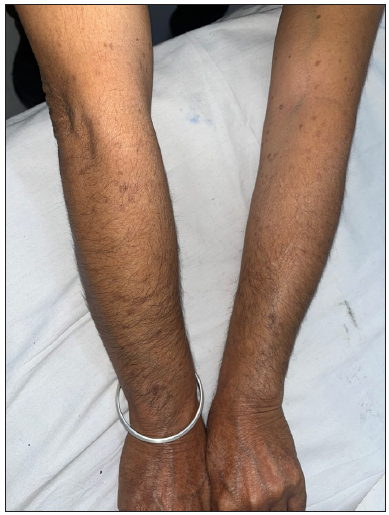
- Forearms post therapy at six months.
Porokeratosis is a disorder of keratinisation; the exact pathogenesis is not known. Recent studies show that germline mutations involving genes encoding like mevalonate kinase (MVK), phosphomevalonate kinase (PMVK), farnesyl diphosphate synthase (FDPS) and mevalonate diphosphate decarboxylase (MVD) play key roles in the disease pathogenesis.4 Loss of function of any of these genes can cause cholesterol deficiency and also accumulation of toxins leading to premature apoptosis and dysregulated keratinocyte differentiation.5 There are few studies which have used topical lovastatin or simvastatin with cholesterol, based on the fact that statins block hydroxymethylglutaryl-CoA (HMG CoA) enzyme which prevents the accumulation of toxic end products and helps cholesterol in accessing keratinocytes for efficient transepidermal incorporation. These studies showed complete resolution of symptoms and partial to near complete resolution of lesions in two months. None of the studies showed complete resolution of lesions.2 Various recent case series published on the role of topical statins in porokeratosis are summarised in Table 1. These findings were similar to our observations.
| Authors | Type of topical statin | Results |
|---|---|---|
| Atzmony L et al.2 | Topical lovastatin + cholesterol, eight patients | Near-complete clearance of DSAP in four weeks and moderate improvement of porokeratosis palmaris et plantaris disseminata lesions and linear porokeratosis. |
| Byth LA et al.3 | Topical simvastatin + cholesterol, eight patients | Improvement in DSAP lesion number, erythema and scaling on treated limbs compared with controls in six weeks. |
| Albanell F et al.6 | Topical simvastatin in two refractory porokeratosis phytotropica patients | In 26 months, 50% reduction in the size of lesion and there was sustained response for two years. |
| Santa Lucia et al.7 | Topical lovastatin+ cholesterol versus topical lovastatin alone, 12 patients in each group | The disease severity decreased by 50% points on the DSAP-GASI; (P < .001) in the lovastatin cholesterol group and 51.4% in the lovastatin group. There was no statistically significant difference between the groups. |
DSAP-GASI: disseminated superficial actinic porokeratosis
It can be hypothesised that by obstructing the defective pathway, toxic end products are prevented from building up and keratinocyte differentiation dysregulation is avoided. Because of this, the lesions have partially resolved. Further exploration regarding proper dosing and duration of topical statins and their efficacy in maintaining long-term remission has to be done. From our observation, we conclude that topical atorvastatin can be a cheap and effective modality in the treatment of extensive porokeratosis leading to significant improvement in the patient’s quality of life. Topical statins with or without cholesterol can be combined with other therapies such as LASERs, which have shown promising outcomes for better cosmetic benefits.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Porokeratosis: A review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr (Engl Ed). 2020;111:545-60.
- [CrossRef] [PubMed] [Google Scholar]
- Topical cholesterol/lovastatin for the treatment of porokeratosis: A pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Topical simvastatin-cholesterol for disseminated superficial actinic porokeratosis: An open-label, split-body clinical trial. Australas J Dermatol. 2021;62:310-13.
- [CrossRef] [PubMed] [Google Scholar]
- Second-hit somatic mutations in mevalonate pathway genes underlie porokeratosis. J Invest Dermatol. 2019;139:2409-11.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130:1136-45.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of porokeratosis ptychotropica with a topical combination of cholesterol and simvastatin. JAMA Dermatol. 2023;159:458-60.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of topical lovastatin plus cholesterol cream vs topical lovastatin cream alone for the treatment of disseminated superficial actinic porokeratosis: A randomized clinical trial. JAMA Dermatol. 2023;159:488-95.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





