Translate this page into:
Topical mTOR (mechanistic target of rapamycin) inhibitor therapy in facial angiofibroma
Correspondence Address:
Debopam Samanta
1 Children's Way, Little Rock, Arkansas, 72202
USA
| How to cite this article: Samanta D. Topical mTOR (mechanistic target of rapamycin) inhibitor therapy in facial angiofibroma. Indian J Dermatol Venereol Leprol 2015;81:540-541 |
Sir,
I read with interest the article by Nath et al. [1] The frequency and characteristics of various cutaneous and extracutaneous manifestations of tuberous sclerosis complex (TSC) were described in a cohort of pediatric patients. I would like to share our experience in the treatment of facial angiofibromas with topical mechanistic target of rapamycin (mTOR) inhibitors. Facial angiofibromas are one of the most common manifestations of tuberous sclerosis. These can cause severe facial disfigurement and psychological problems. A new tool, the facial angiofibroma severity index has also been developed to evaluate the grade of erythema and the size and extent of angiofibromas. [2] Surgical therapies such as excision, dermabrasion, radiosurgery and lasers are the most commonly employed modalities to treat facial angiofibromas. However, these procedures have obvious drawbacks which include the associated adverse effects such as pain, scars from the procedure, requirement of anesthesia, and frequent recurrence requiring periodic and repeated procedures.
Tuberous sclerosis complex is caused by a mutation in either the TSC1 (protein product hamartin) or the TSC2 (protein product tuberin) gene, leading to up-regulation of mTOR, causing uncontrolled cellular growth, proliferation and protein synthesis. For last several years, mTOR inhibitors such as rapamycin and everolimus have been used to treat subependymal giant cell astrocytoma and renal angiomyolipoma, associated with tuberous sclerosis complex. Topical therapy has been reported to have a beneficial effect in facial angiofibromas, where systemic therapy is not necessary. [3] In our multispecialty tuberous sclerosis complex center at Arkansas Children′s Hospital, we routinely offer treatment with topical sirolimus and everolimus for facial angiofibromas. These topical agents are easy to prepare in a compounding pharmacy and have a reasonable shelf-life [Table - 1]. Several patients at our center have had a significant and dramatic benefit from this treatment [Figure - 1]. Most patients have improvement within the first 4-8 weeks of daily administration. Though, recurrence of lesions is seen commonly in non-compliant patients, the severity of angiofibromas is usually much less prominent in postpubertal subjects. These topical agents do not get absorbed in the blood and are free of systemic side-effects. Skin burning, erythema, irritation and dryness can be seen, possibly due to preservatives and additives and can be avoided with a skin protective agent. In resource-poor settings, there is some potential drawback of its regular use such as the expense of the medicine, need of standardized dispensing and long and undetermined duration of therapy. In future, rigorous studies are needed to decide the duration and requirement of maintenance therapy to prevent relapse of angiofibromas. Dissemination of this information and widespread use of these topical agents may decrease the psychological burden of facial angiofibroma, at least to some extent.

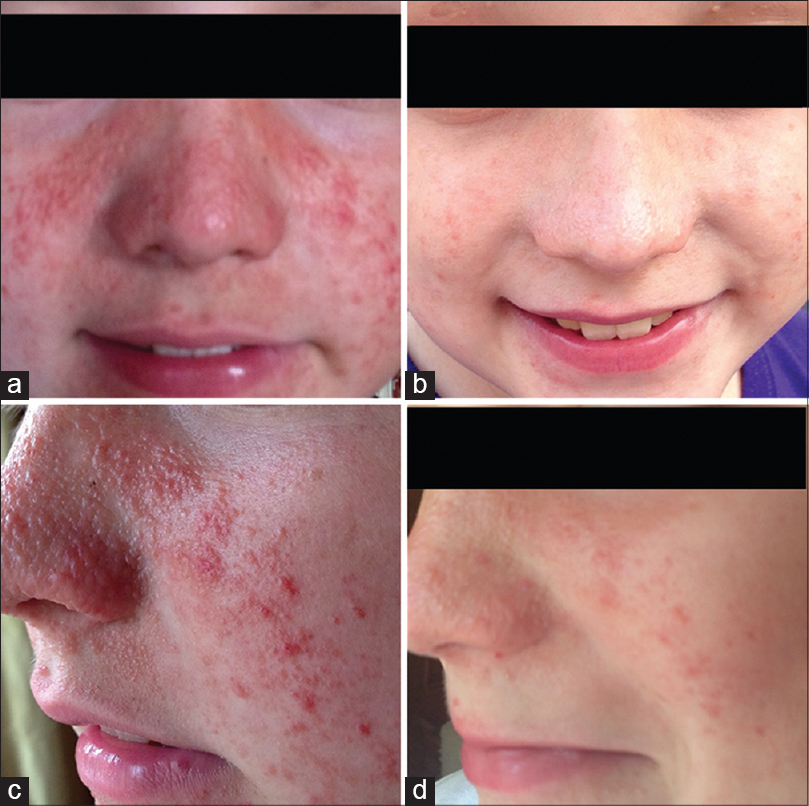 |
| Figure 1: Before (a and c) and 2 months after (b and d) topical mechanistic target of rapamycin inhibitor therapy |
Acknowledgments
The study was done at Arkansas Children′s Hospital, Little Rock, Arkansas. We thank Jacqueline Edens, TSC clinic coordinator, for her exceptional dedication in managing these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Nath J, Dubey A, Pavan R. Analysis of twenty pediatric cases of tuberous sclerosis complex: Are we doing enough? Indian J Dermatol Venereol Leprol 2015;81:23-8.
[Google Scholar]
|
| 2. |
Salido-Vallejo R, Ruano J, Garnacho-Saucedo G, Godoy-Gijón E, Llorca D, Gómez-Fernández C, et al. Facial Angiofibroma Severity Index (FASI): Reliability assessment of a new tool developed to measure severity and responsiveness to therapy in tuberous sclerosis-associated facial angiofibroma. Clin Exp Dermatol 2014;39:888-93.
[Google Scholar]
|
| 3. |
Dill PE, De Bernardis G, Weber P, Lösch U. Topical everolimus for facial angiofibromas in the tuberous sclerosis complex. A first case report. Pediatr Neurol 2014;51:109-13.
[Google Scholar]
|
Fulltext Views
4,158
PDF downloads
2,658





