Translate this page into:
Topical timolol in PHACES syndrome: Is it safe?
2 Department of Radiology, University College of Medical Sciences, GTB Hospital, University of Delhi, New Delhi, India
Correspondence Address:
Deepika Pandhi
Department of Dermatology and STD, University College of Medical Sciences, GTB Hospital, University of Delhi, New Delhi - 110 095
India
| How to cite this article: Pandhi D, Jakhar D, Tandon A, Singal A. Topical timolol in PHACES syndrome: Is it safe?. Indian J Dermatol Venereol Leprol 2018;84:488-491 |
Sir,
PHACE(S) syndrome is a congenital neurocutaneous entity that includes posterior fossa abnormalities, hemangiomas, arterial abnormalities, cardiac abnormalities, eye abnormalities and sternal cleft/supraumbilical raphe.[1] The syndrome has a striking predilection for the female gender.[1] Presence of a large segmental hemangioma in a child should prompt the clinician to undertake a thorough workup of the patient for the above-mentioned associations.[2] Herein we report a 3-month-old female child with PHACE(S) syndrome with a prominent cerebrospinal fluid cleft communicating with the posterior aspect of 4th ventricle (a variant of Dandy-Walker malformation) and an absent right internal carotid artery.
A 3-month-old female child, born by a full-term vaginal delivery, fourth in the order of birth, born of a consanguineous marriage, presented to the outpatient department, with the complaint of red raised plaques over the entire right upper limb, right side of upper chest, face involving both eyelids, right side of upper lip, philtrum, nose, the frontal and temporal regions of the scalp [Figure - 1] and [Figure - 2]. Plaques over the forehead, right cheek and eyelids showed ulceration, hemorrhagic crusting and redness; swelling and discharge from both eyes was also evident [Figure - 1]. There was history of application of an unknown topical medication over the plaques, leading to ulceration. The child was not immunized adequately, while her developmental milestones were appropriate for age.
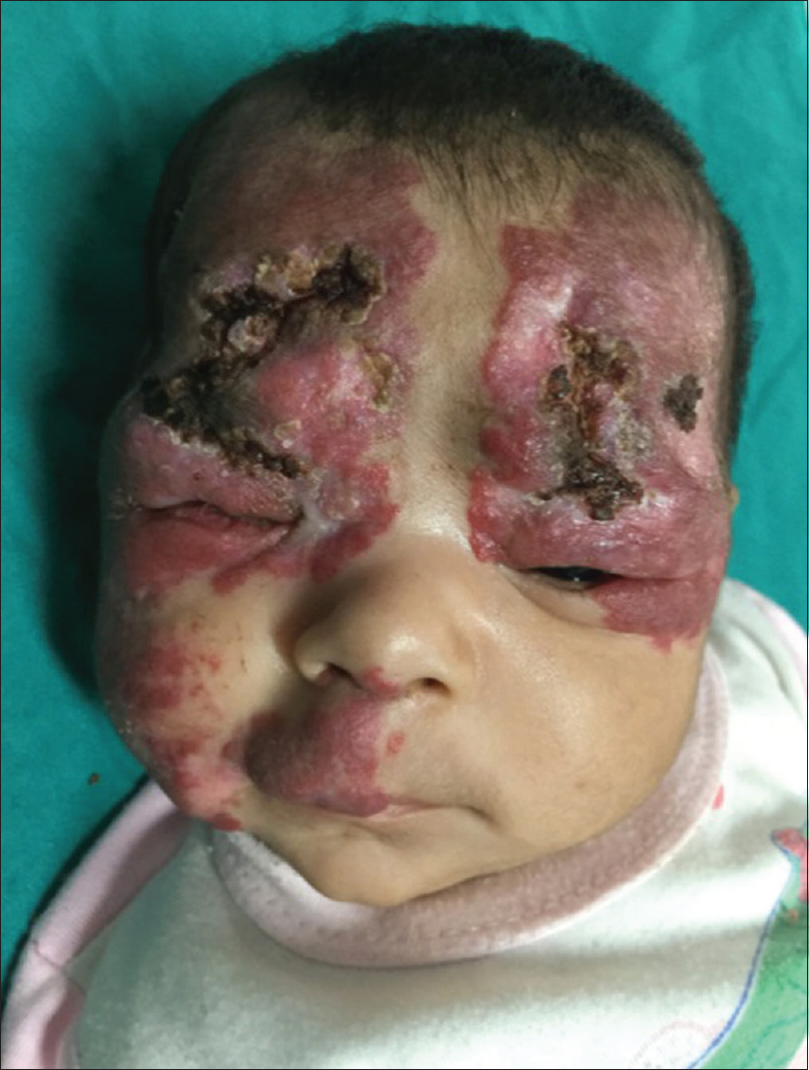 |
| Figure 1: Hemangioma involving upper face including peri-orbital regions, cheek, forehead, frontal and right temporal scalp and philtrum of nose. Note the ulceration and hemorrhagic crusting and discharge from the eyes |
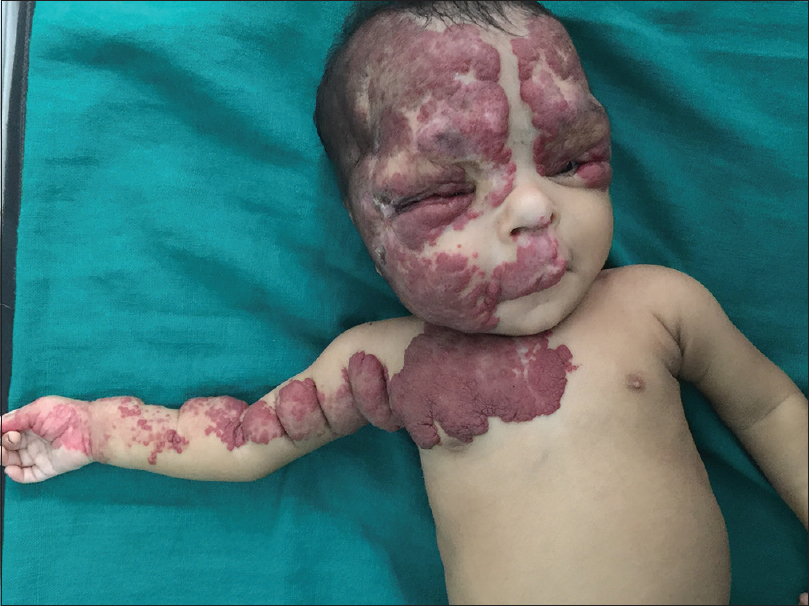 |
| Figure 2: Hemangioma involving bilateral upper face including peri-orbital regions, right cheek, philtrum of nose, right chest, arm and hand |
Oral examination revealed the presence of a hemangioma over the right hard palate, extending upto the floor of the nose [Figure - 3]. Ophthalmological evaluation showed swelling of bilateral eyelids, with disruption of the normal anatomy of anterior and posterior lamellae.
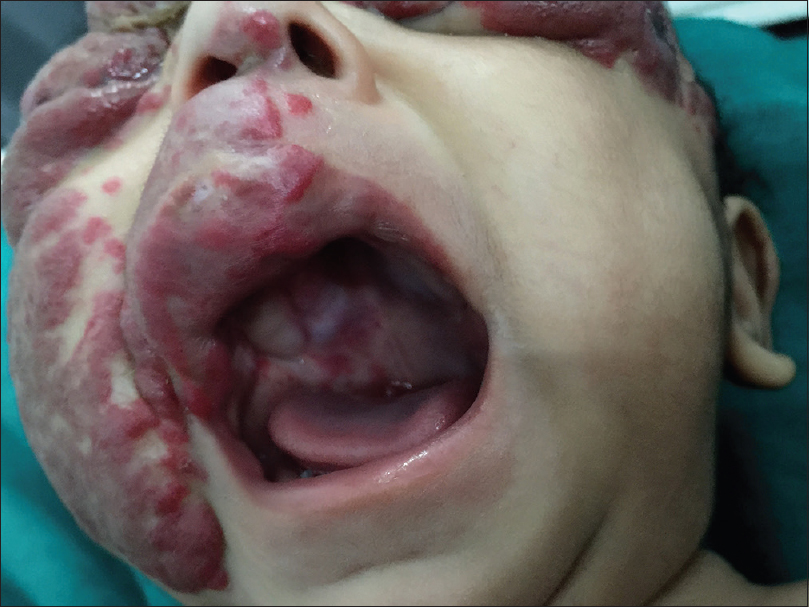 |
| Figure 3: Hemangioma present over the right hard palate |
Hematological, biochemical parameters and thyroid function tests and serum cortisol levels were within normal limits. Electrocardiogram, echocardiogram, ultrasonography of abdomen and cranium showed no abnormality. However, magnetic resonance imaging of the brain showed extensive soft tissue thickening with signal alteration and areas of flow void involving upper face including periorbital regions, cheek, forehead, bilateral frontal and right temporal scalp, which was consistent with soft tissue hemangioma. There was involvement of preseptal and postseptal compartment of bilateral orbits, with extension in the retrobulbar space and encasement of the muscle cone bilaterally. Also noted were a posterior fossa anomaly with hypoplastic right cerebellar hemisphere and vermis, and prominent cerebrospinal fluid cleft communication with the posterior aspect of 4th ventricle. The right internal carotid artery was absent [Figure - 4].
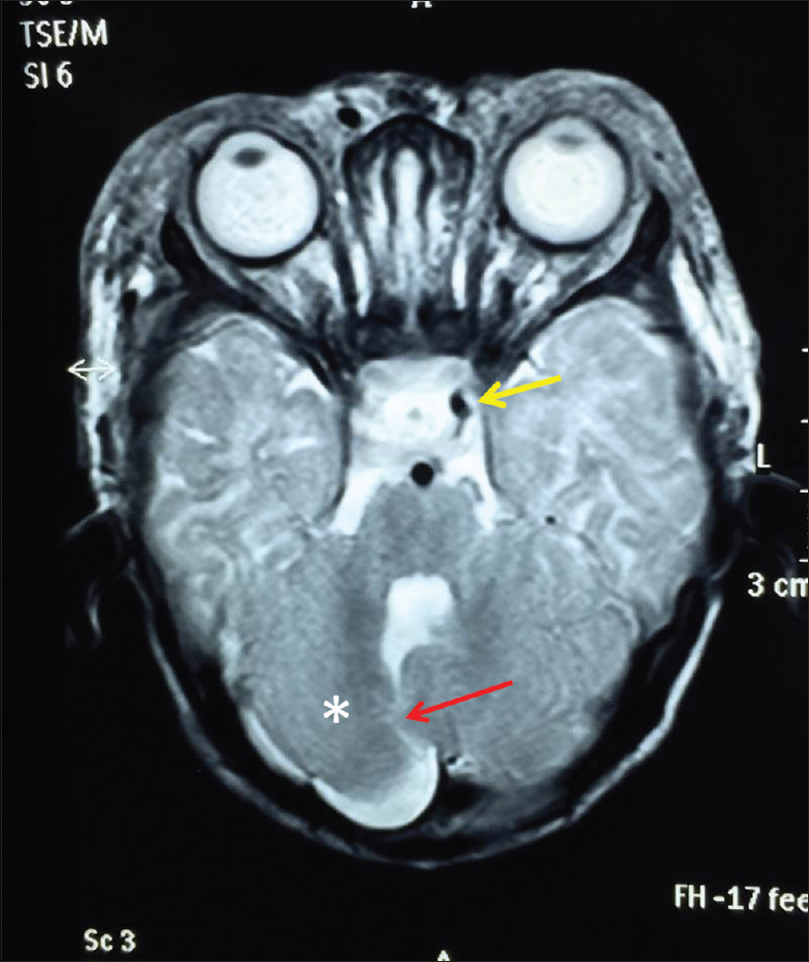 |
| Figure 4: Axial T2W magnetic resonance image reveals subcutaneous thickening with few flow voids and a posterior fossa anomaly; hypoplastic right cerebellum (*) with an abnormal cerebrospinal fluid cleft (red arrow) communicating with posterior aspect of 4th ventricle and absence of right internal carotid artery (Note: left internal carotid artery is seen as dark dot- yellow arrow and right internal carotid artery dark dot is absent). This constellation of findings was consistent with PHACES syndrome |
We initiated oral prednisolone (3.3 mg/kg), along with systemic and topical antibiotics including tobramycin eye drops. The crusted lesions healed within a week with resolution of the discharge and redness of the eyes. After 15 days, topical timolol (drops 0.5%), 1 drop per cm 2 (4 drops per lesion) twice daily, without occlusion, was added for the non-ulcerated hemangioma over the trunk. The patient's mother was counselled regarding the possible side-effects such as bradycardia, hypotension, bronchospasm, peripheral vasoconstriction, weakness and fatigue, sleep disturbances, gastrointestinal disturbance and hypoglycemia; and the importance of not exceeding the recommended dose was stressed upon. Hyperkalemia (6.5 mEq/L) was noted after 2 weeks while carrying out biochemical investigations to monitor therapy. The blood pressure and other biochemical investigations, including blood sugar levels were unremarkable. Electrocardiograhic changes in the form of peaked T waves, S-T segment depression and a widened QRS complex, corroborated hyperkalemia. Timolol drops were withdrawn, and after pediatric consultation, the patient was given parenteral calcium gluconate (10%) at a dose of 40 mg/kg, which was repeated every 6 hours. Hyperkalemia resolved and the patient was continued on oral prednisolone and tobramycin eye drops. Regression of hemangioma was evident at 2-months' follow-up [Figure - 5]. The patient was discharged on the same treatment, with the plan to follow-up at 2 week intervals, but was subsequently lost to follow-up.
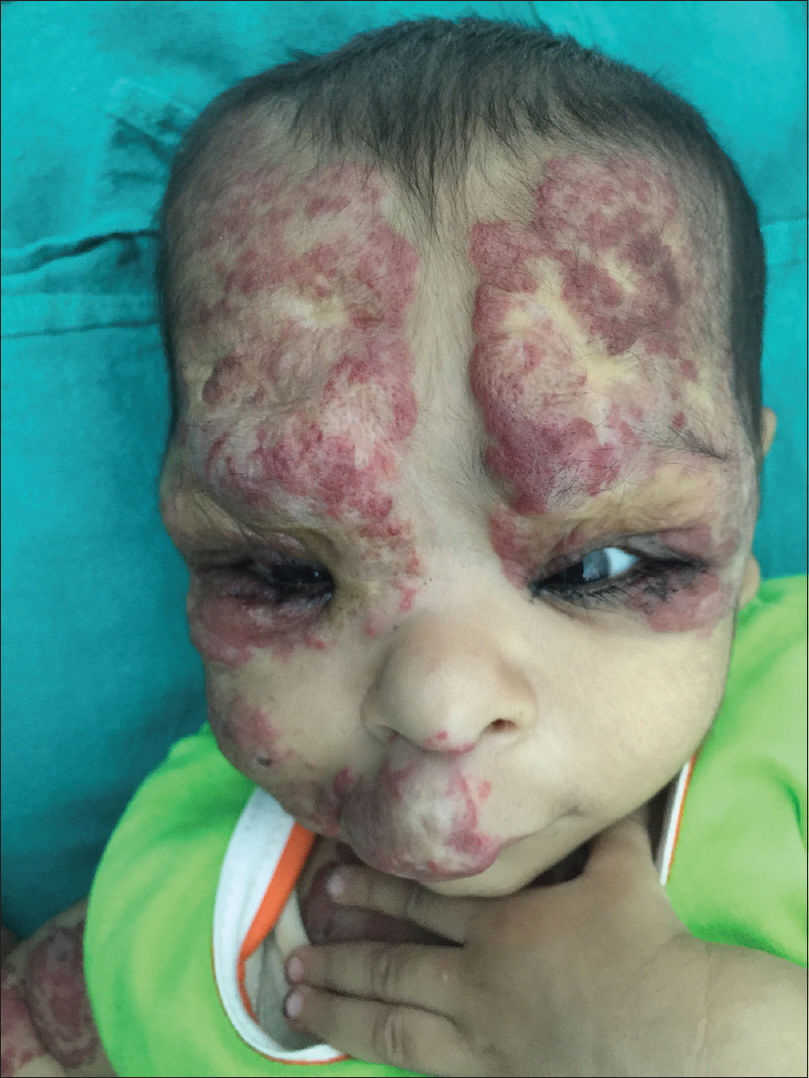 |
| Figure 5: Regression of lesions after 2 months of oral corticosteroids |
PHACE(S) syndrome (OMIM 606519) is an extremely rare, neurocutaneous form of syndromal infantile hemangioma. The diagnosis of PHACES syndrome requires the presence of facial hemangioma of greater than 5 cm dimension, and one major or two minor criteria.[3] Major criteria include anomaly of major cerebral arteries, posterior fossa anomaly such as Dandy–Walker complex, aortic arch anomaly, posterior segment ocular anomalies and sternal defects. The minor anomalies are persistent embryonic artery other than trigeminal artery, intracranial hemangioma or midline anomaly or neuronal migration disorder, ventral septal defects, anterior segment ocular anomalies and hypopituitarism.[3] In our case, the facial hemangioma was more than 5 cm, and two major criteria were fulfilled, viz., anomaly of major cerebral arteries in the form of absent right internal carotid artery and posterior fossa anomaly with hypoplastic right cerebral hemisphere and vermis and prominent cerebrospinal fluid cleft communication with the posterior aspect of fourth ventricle.
Standardization of a therapeutic protocol for PHACES syndrome is challenging; various treatment modalities include oral, topical and injectable corticosteroids, oral and topical beta-blockers, and miscellaneous agents such as vincristine, interferon-α, imiquimod and pulse-dye lasers. From 1960s onwards, corticosteroids emerged as the traditional treatment option for cutaneous hemangiomas.[4] However, side-effects such as hypertension, adrenal suppression, hyperglycemia, growth delay, cushingoid features and behavioral problems limit its use, especially when treatment is required for a prolonged duration. After the first report in 2008 regarding the use of propranolol,[5] a nonselective beta-blocker, for the treatment of infantile hemangiomas, there have been a number of case reports and case series supporting the successful use of beta-blockers for infantile hemangiomas and PHACE syndrome.[6],[7] Propranolol acts, probably, by a combination of factors, namely, vasoconstriction, inhibition of angiogenesis and induction of apoptosis.[8] However the efficacy, optimum dosage, duration of treatment, monitoring during the therapy and side-effects are still not known entirely. Reported side-effects include hypotension, hypoglycemia, bradycardia, bronchospasm, somnolence and gastrointestinal disturbances. In PHACES syndrome, there is a potential contraindication for the use of propranolol, especially if it is associated with an underlying vasculopathy.[9] In such cases, systemic beta-blockers pose a risk of stroke and brain ischemia. In the latest consensus conference on the use of propranolol in infantile hemangioma, PHACES syndrome associated with cerebrovascular anomalies was stratified as high-risk group for initiating propranolol therapy.[10]
Because our patient had a high risk for systemic propranolol due to absent right internal carotid artery, oral corticosteroids and topical timolol were initiated. In a series of 32 infants of PHACES syndrome, Metry et al. reported 7 patients with magnetic resonance imaging findings of severe, long-segment narrowing or non-visualization of major cerebral or cervical vessels and absence of anatomic evidence of collateral circulation.[7] They concluded that systemic beta-blockers have to be used with caution in these high-risk patients given the increased probability of stroke in such patients.[7]
Although no current guidelines exist on the usage of topical timolol, it is being used increasingly, as it is readily available in an ophthalmic formulation, and has a good safety profile and efficacy.[11] However, there are concerns regarding the systemic absorption of topical timolol.[12] The side-effect profile of topical beta-blockers could be same as that of oral medications; there is a lack of studies on the pharmacokinetics of topical timolol.[12] Our patient developed hyperkalemia within 2 weeks of starting topical timolol, associated with electrocardiogram changes, and hyperkalemia resolved after stopping topical timolol. Hyperkalemia with use of beta-blockers is rarely observed.[13] These agents can cause selective hyperkalemia by preventing beta-receptor-mediated hepatic and skeletal muscle potassium uptake.[13] Suppression of renin and aldosterone release by beta-blockers is another possible mechanism for impaired potassium homeostasis. Hyperkalemia with topical timolol has been described in a patient on long-term topical timolol for glaucoma, when oral corticosteroids were added for radiation pneumonitis. The authors postulated that the high catabolic load of potassium induced by corticosteroids could not be cleared due to the effect of concomitant topical timolol applied to one eye.[13] This report emphasizes that the risk for hyperkalemia with topical timolol, however minute, does exist and is probably exacerbated in patients on oral corticosteroids. Furthermore, there is no literature to support or reject the efficacy and safety of oral corticosteroids in patients with absent ICA. Therefore, until standard guidelines become available, a short course of oral corticosteroids can be initiated, with close monitoring. Regarding topical beta-blockers, patients should be monitored on the same recommendations as for systemic therapy. Finally, yet importantly, parents should also be prognosticated regarding language and speech disorders, learning disabilities and cognitive disabilities associated with cerebellar hypoplasia.[14]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given her consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal patient identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 1996;132:307-11.
[Google Scholar]
|
| 2. |
Bronzetti G, Giardini A, Patrizi A, Prandstraller D, Donti A, Formigari R, et al. Ipsilateral hemangioma and aortic arch anomalies in posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta, and cardiac defects and eye abnormalities (PHACE) anomaly: Report and review. Pediatrics 2004;113:412-5.
[Google Scholar]
|
| 3. |
Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, et al. Consensus statement on diagnostic criteria for PHACE syndrome. Pediatrics 2009;124:1447-56.
[Google Scholar]
|
| 4. |
Cohen SR, Wang CI. Steroid treatment of hemangioma of the head and neck in children. Ann Otol Rhinol Laryngol 1972;81:584-90.
[Google Scholar]
|
| 5. |
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51.
[Google Scholar]
|
| 6. |
Manunza F, Syed S, Laguda B, Linward J, Kennedy H, Gholam K, et al. Propranolol for complicated infantile haemangiomas: A case series of 30 infants. Br J Dermatol 2010;162:466-8.
[Google Scholar]
|
| 7. |
Metry D, Frieden IJ, Hess C, Siegel D, Maheshwari M, Baselga E, et al. Propranolol use in PHACE syndrome with cervical and intracranial arterial anomalies: Collective experience in 32 infants. Pediatr Dermatol 2013;30:71-89.
[Google Scholar]
|
| 8. |
Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269-74.
[Google Scholar]
|
| 9. |
Drolet BA, Dohil M, Golomb MR, Wells R, Murowski L, Tamburro J, et al. Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics 2006;117:959-64.
[Google Scholar]
|
| 10. |
Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics 2013;131:128-40.
[Google Scholar]
|
| 11. |
Coppens G, Stalmans I, Zeyen T, Casteels I. The safety and efficacy of glaucoma medication in the pediatric population. J Pediatr Ophthalmol Strabismus 2009;46:12-8.
[Google Scholar]
|
| 12. |
Berk DR, Lehman PA, Franz TJ, Blunt JR, Bayliss SJ. On topical timolol gel-forming solution for infantile hemangiomas. Pediatr Dermatol 2013;30:160-1.
[Google Scholar]
|
| 13. |
Swenson ER. Severe hyperkalemia as a complication of timolol, a topically applied beta-adrenergic antagonist. Arch Intern Med 1986;146:1220-1.
[Google Scholar]
|
| 14. |
Poretti A, Limperopoulos C, Roulet-Perez E, Wolf NI, Rauscher C, Prayer D, et al. Outcome of severe unilateral cerebellar hypoplasia. Dev Med Child Neurol 2010;52:718-24.
[Google Scholar]
|
Fulltext Views
3,479
PDF downloads
1,446





