Translate this page into:
Two cases of subungual glomus tumor
Correspondence Address:
P S Murthy
Department of Dermatology and STD, Command Hospital Air Force, Bangalore-560 007
India
| How to cite this article: Murthy P S, Rajagopal R, Kar P K, Grover S. Two cases of subungual glomus tumor. Indian J Dermatol Venereol Leprol 2006;72:47-49 |
Abstract
Glomus tumors are uncommon, small, painful, and usually benign hamartomas arising from the arterial end of the glomus body. They often present early in the subungual stage because of intense pain. Two female patients with subungual glomus tumor are reported here. The intense pain associated with this tumor had led to disuse atrophy of the upper limb in one case. Hildreth's sign and Love's test were positive in both, but imaging did not help in preoperative diagnosis. Tumors were resected by transungual approach, leaving a 3-mm-wide margin. There was no recurrence after 1-year follow-up in both instances.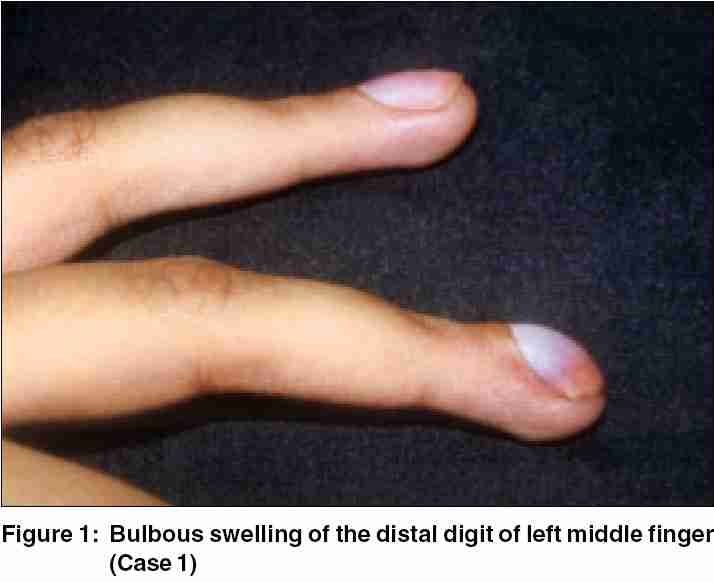 |
 |
INTRODUCTION
The normal glomus body is an arteriovenous shunt related to thermoregulation, located mostly in the digits. The glomus tumor is an uncommon hamartoma composed of endothelium-lined vascular spaces (the Sucquet-Hoyer canal) surrounded by glomus cells,[1],[2] arising from the arterial end of the glomus body. Wood first described them in 1812.[1] They have been reported from several organs. The majority of glomus tumors are small, benign neoplasms that occur in the dermis or subcutis of the extremities.[3] These tumors can either be solitary or multiple. The solitary subungual tumors are common in females. We report on two cases of subungual glomus tumors, both in females, one of which was so painful that it led to wasting of the hand and forearm owing to disuse.
CASE REPORT
Case 1
A 21-year-old female presented with a painful, 12-year-old swelling of distal digit of the left middle finger. The swelling was intensely painful on exposure to cold, pressure, and accidental trauma. She had earlier been operated for the same complaint but swelling and pain recurred within 6 months. She had been avoiding use of the left hand for fear of accidental trauma as even the slightest injury caused excruciating pain. She had given up her occupation as a typist. Dermatological examination revealed bulbous terminal digit of left middle finger [Figure - 1] with deformed, slightly bluish nail plate and extreme tenderness over the nail. Hildreth′s sign and Love′s test were positive, but cold-sensitivity could not be elicited. There was wasting of the left hand and forearm owing to disuse. X-ray did not reveal any abnormality. Transungual surgical approach revealed shiny encapsulated tumor, which was excised in toto and biopsy confirmed glomus tumor.
Case 2
A 25-year-old pregnant primigravida presented with an 8-year history of excruciating spontaneous episodic shooting pain over the terminal digit of the right index finger. The pain lasted for few seconds at a time, but increased in intensity. It had become more frequent during the pregnancy. Tapping the finger or accidental injury set off flashes of pain. This was relieved partially by elevating the limb. Examination revealed slight bulbous swelling, but dipping the finger in ice cold water did not elicit pain. X-ray and high-resolution CT did not reveal any abnormality. Transungual surgical approach revealed a 2-mm-diameter pearly, shiny, encapsulated swelling that was removed with a 3-mm-wide margin. Histopathological examination confirmed the clinical and perioperative diagnosis of glomus tumor.
DISCUSSION
Glomus tumors are extremely painful and present early. Clinically, solitary lesions are characterized by a reddish-purple discoloration of the lesion, paroxysmal pain, tenderness, and cold-sensitivity. The lesion is usually located on the extremities, especially the nail bed, as seen in both of our patients, but may occur on other parts of the body. Pain can be of severe nature, leading to disuse atrophy of the limb. Multiple lesions are more common in males. Multiple glomus tumors, also known as glomangiomas, are inherited by autosomal-dominant pattern with incomplete penetrance. They are less solid and have the appearance of a hemangioma, and are said to be less painful. Very rarely, platelet sequestration may be a concern in patients with widely disseminated lesions. Although glomus tumors are thought to arise from glomus cells, these tumors have been observed in extracutaneous locations that are not known to contain glomus cells. Intra-oral glomus tumors have also been reported.[4] Occasional glomus tumors may show unusual clinical features such as large size, deep soft tissue, or visceral location, infiltrative growth pattern, or multicentricity. [5],[6],[7] There are very few case reports of clinically malignant glomus tumors.[5]
Two clinical findings are described, particularly in relation to the painful subungual solitary glomus tumors. They are the Hildreth′s sign and Love′s test. Hildreth′s sign is disappearance of pain after application of a tourniquet proximally on the arm. One study[8] assessed Hildreth′s sign and found 92% sensitivity and 91% specificity with a positive predictive value of 92% and a negative predictive value of 91%. Love′s test consists of eliciting pain by applying pressure to a precise area with the tip of a pencil. In one study[9] comprising 18 patients, cold-sensitivity and Hildreth′s tests have sensitivities of 100% and 77.4%, respectively, and specificity of 100%. Love′s test had a sensitivity of 100%. Love′s and Hildreth′s tests showed 78% accuracy, whereas the cold-sensitivity test was 100% accurate. Hildreth′s sign and Love′s test were positive in both of our cases, but cold-sensitivity was not present in either case. Routine laboratory studies have no role in diagnosis of glomus tumor. Standard magnetic resonance imaging (MRI) can be used to detect glomus tumors, but high-resolution MRI assessed tumor characteristics more accurately.[10] High-resolution CT was unhelpful in one of our cases.
Histologically, the glomus tumor shows numerous small, vascular lumina, lined with a single layer of flattened endothelial cells. The vascular spaces were surrounded by clusters of cells characterized by a faintly eosinophilic cytoplasm and a large, pale nucleus. Immuno-histochemical studies[11] showed that glomus tumor cells stained positively for smooth-muscle actin. In some cases, the glomus tumor cells weakly expressed desmin. They were not reactive with Ulex europaeus agglutinin-I and anti-factor-VIII-related antigen, whereas only the endothelial cells were reactive with them. The tumor cells as well as vascular endothelial cells among the tumor cells expressed CD34 in five of the cases.[11]
The treatment of choice for solitary glomus tumors is surgical excision. For multiple glomus tumors, excision may be more difficult because of their poor circumscription and the large number of lesions. Excision should be limited to symptomatic lesions. Subungual glomus tumors are more difficult to treat because they are small, sometimes total eradication requires several procedures.[1],[2] Local recurrences usually represent inadequate excision because infiltrative growth and local invasion of the capsule around the glomus tumor have been reported to occur in only 1-2% of cases.[2] To avoid recurrence of pain, it is important that the accurate preoperative localization of the tumor and a complete extirpation should be performed. To avoid nail deformity, it is better to apply a periungual approach for tumors developing in the peripheral region, and a transungual approach followed by meticulous repair of the nail bed for tumors developing in the central region.[12] In both of our cases we could not localize the lesions preoperatively, and hence preferred the transungual approach. The tumors were excised completely and though there was no recurrence of pain on follow-up for 1 year, the subsequent nail plate was deformed. Interestingly, there is an earlier case report of glomus tumor leading to disuse atrophy.[13]
| 1. |
Dawber RP, Baran R. Disorders of nails. In : Champion RH, Burton JL, Ebling FJG, editors. Rook/Wilkinson/Ebling Textbook of Dermatology. 5th ed. Oxford: Blackwell Science; 1992. p. 2497-532.
[Google Scholar]
|
| 2. |
Heys SD, Brittened J, Atkinson P, Eremin O . Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg 1992;79:345-7.
[Google Scholar]
|
| 3. |
Enzinger FM, Weiss SW. Soft Tissue Tumors. 2nd ed. St. Louis: Mosby; 1988. p. 422-32.
[Google Scholar]
|
| 4. |
Geraghty JM, Thomas RW, Robertson JM, Blundel JW . Glomus tumour of the palate: case report and review of the literature. Br J Oral Maxillofac Surg 1992;30:398-400.
[Google Scholar]
|
| 5. |
Gould EW, Manivel JC, Albores-Saavedra J, Monteforte H. Locally infiltrative glomus tumours and glomangiosarcomas: a clinical, ultrastructural and immunohistochemical study. Cancer 1990;65:310-8.
[Google Scholar]
|
| 6. |
Kiyosawa T, Umebayashi Y, Nakayama Y, Soeda S. Hereditary multiple glomus tumours involving glans penis. A case report and review of literature. Dermatol Surg 1995;21:895-9.
[Google Scholar]
|
| 7. |
Lumley JS, Stansfiels AG. Infiltrating glomus tumour of lower limb. BMJ 1972;1:484-5.
[Google Scholar]
|
| 8. |
Giele H. Hildreth's test is a reliable clinical sign for the diagnosis of glomus tumours. J Hand Surg 2002;27:157-8.
[Google Scholar]
|
| 9. |
Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg 2002;27:229-31.
[Google Scholar]
|
| 10. |
Drape JL, Idy-Peretti I, Goettmann S, Guerin-Surville H, Bittoun J. Standard and high resolution magnetic resonance imaging of glomus tumors of toes and fingertips. J Am Acad Dermatol 1996;35:550-5.
[Google Scholar]
|
| 11. |
Hatori M, Aiba S, Kato M, Kamiya N, Kokuben S. Expression of CD 34 in glomus tumors. Tohoku J Exp Med 1997;182:241-7.
[Google Scholar]
|
| 12. |
Takata H, Ikuta Y, Ishida O, Kimori K. Treatment of subungual glomus tumour. Hand Surg 2001;6:25-7.
[Google Scholar]
|
| 13. |
Garg BR, Lal Sardari. Glomus tumours with disuse atrophy. Indian J Dermatol Venereol Leprol 1973;39:270-1.
[Google Scholar]
|
Fulltext Views
6,627
PDF downloads
2,380





