Translate this page into:
Ultraviolet A1 phototherapy: One center's experience
2 Department of Dermatology and Photobiology, Ninewells University Hospital, Dundee, DD1 9SY, Scotland, UK
Correspondence Address:
Sasi Kiran Attili
Visakha Institute of Skin and Allergy, Coastal Battery Road, Maharanipeta, Visakhapatnam - 530 002, Andhra Pradesh
| How to cite this article: Attili SK, Dawe RS, Ibbotson SH. Ultraviolet A1 phototherapy: One center's experience. Indian J Dermatol Venereol Leprol 2017;83:60-65 |
Abstract
Background: Ultraviolet A1(UVA1) phototherapy is increasingly being used in the treatment of morphea, atopic dermatitis, lupus and some other recalcitrant dermatoses. We present a retrospective review of our experience with this modality. Aim: To evaluate the treatment response rates for various dermatoses and adverse effects of UVA1 phototherapy. Methods: We reviewed phototherapy notes along with electronic and/or paper case records for all patients treated with UVA1 phototherapy from October 1996 to December 2008. Results: A total of 269 patients (outcomes available for 247) had 361 treatment courses (treatment data available for 317 courses) over this period. We found phototherapy to be beneficial in 28 (53%) of 53 patients with atopic dermatitis and 19 (51%) of 37 patients with morphea. A beneficial outcome was recorded in all six (100%) cases of urticaria and six (85.7%) of seven patients treated for a polymorphic light eruption. Benefit was also recorded in systemic lupus erythematosus (8 (44.4%) of 18), lichen sclerosus (6 (42.9%) of 14), mastocytosis (2 (33.3%) of 6), necrobiosis lipoidica (4 (30.8%) of 13), granuloma annulare (2 (25%) of 8), scleroderma (2 (22.2%) of 9) and keloids (1 (7.7%) of 13). Overall, treatment was well tolerated with no patients having to stop treatment due to adverse effects. Limitations: This is a retrospective study with no control group. Subjective/recall bias is quite possible as a number of patients were followed up over the phone. Conclusions: Our data suggest that ultraviolet A1 can be considered for the treatment of selected dermatoses. However, long-term malignancy risk is as yet unknown.Introduction
In recent years, the place of UVA1 phototherapy in the management of a range of chronic skin diseases, particularly atopic dermatitis, sclerosing/fibrosing dermatoses and systemic lupus erythematosus has been investigated.[1],[2],[3],[4],[5],[6] The exact mechanism by which UVA1 phototherapy helps in various diseases is also currently under investigation. It is believed to mainly exert its effects via oxygen-dependent indirect mechanisms through absorption by endogenous photodynamic photosensitizers including lipids and proteins. However, the molecules that absorb UVA1 radiation leading to its biological effects have not been conclusively identified. The effects of UVA1-generated singlet oxygen on T-lymphocytes, Langerhans cells and mast cells are likely to contribute to its efficacy in inflammatory disease. The greater susceptibility of malignant T-cells to free radical damage may explain the efficacy of UVA1 in mycosis fungoides. UVA1-induced singlet oxygen and hydrogen peroxide modulate the activity of matrix metalloproteinases produced by fibroblasts. Collagenase mRNA is upregulated in morphea fibroblasts after UVA1 irradiation, a mechanism that is thought to underlie the efficacy of UVA1 in sclerotic dermatoses.[6]
UVA1 phototherapy is currently being offered at four centers in the UK and it is uncertain if it should be developed in other dermatology departments.[6] We commenced UVA1 phototherapy in 1996 using the TL-10 fluorescent whole-body UVA1 stand-up unit (ultraviolet 7001, output ≈20 mW/cm [2]). In 2002, we acquired a portable metal-halide unit (Dr. Hönle, Dermalight UltrA 1, output ≈35–40 mW/cm [2] measured at a distance of 30 cm using a Waldmann ultraviolet meter) for delivering higher outputs to localized areas (up to 20 cm diameter). A high-output metal halide UVA1 whole-body treatment unit (Sellamed 24000® bed, Sellas Medical Devices, GmbH, Gevelsburg, Germany) was acquired in 2004. We present a retrospective review of our experience.
Methods
All patients treated from October 1996 to December 2008 were included in the review. Patients were identified from the Ninewells section of the National Managed Clinical Network for Phototherapy in Scotland (Photonet) database (Photosys). We reviewed phototherapy notes along with electronic and/or paper case records for all patients. Data collected included age, sex, skin phototype, diagnosis, treatment parameters, minimal erythema dose, maximum tolerated dose, total dose, frequency of treatments, adverse effects and outcome. Patients who discontinued therapy due to reasons unrelated to treatment were excluded from the analysis.
The main indications for UVA1 phototherapy in our department include dermatoses recalcitrant or unresponsive to traditional phototherapy regimes (notably atopic dermatitis), photodermatoses not/less sensitive within the UVA1 spectrum (such as lupus) and sclerosing/fibrosing dermatoses. Topical and systemic therapies were allowed to continue with UVA1, hence some patients got UVA1 alone while some got UVA1 along with their topical or systemic therapy. However, patients on potentially phototoxic drugs or on steroid-sparing immunosuppressants were not considered for therapy with UVA1.
Though a number of studies report UVA1 use at standard doses based on skin phototype,[7] we prefer to perform minimal erythema dose testing routinely before starting phototherapy in our patient population with predominantly skin types I–III. Patients were usually started at 50% of their minimal erythema dose, though this was lowered for photodermatoses such as lupus. Treatment was continued at dose increments of 20% for a trial of 15 sessions. Most patients who noticed some benefit were advised to continue for another 15 sessions (or until no further benefit was noticed over 4–5 consecutive sessions).
Grading of post-treatment erythema was performed as for minimal erythema dose grading, on a scale of 1–4 (where 1 indicates just perceptible erythema, 2, well-defined erythema, 3, erythema and edema and 4, erythema, edema and blistering). If patients developed erythema during treatment, the dose was reduced to the previously tolerated dose and subsequent increments reduced to 10%. Similarly, if one or more treatments were missed, the dose was maintained or reduced. Patients from outside our catchment area (Tayside) were treated as inpatients. Such patients were treated daily (Monday-Friday) while most other patients were treated 3 times/week.
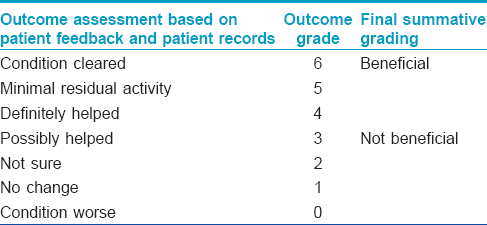
Treatment outcome data were obtained from patient case notes. In addition, where possible, patients were contacted by telephone and their personal opinion sought. Treatment outcomes were graded on a 7-point scale [Table - 1], further classified as “beneficial” or “not beneficial.”
Results
We identified 269 patients from the database. These patients had had a total of 361 treatment courses, a number of patients having had repeat courses. The majority (71%) of patients were female and of skin phototypes I–III (89%) with a median age of 44 years (range, 10–83 years). We identified 40 different diseases from the database [Figure - 1] and [Table - 2]. A third of patients treated had either atopic dermatitis (58 of 269) or morphea (41 of 269). UVA1 was preferred for sclerosing diseases and lupus erythematosus, while for most other diseases it was considered when patients failed to respond to ultraviolet B and/or psoralens and ultraviolet A phototherapy.
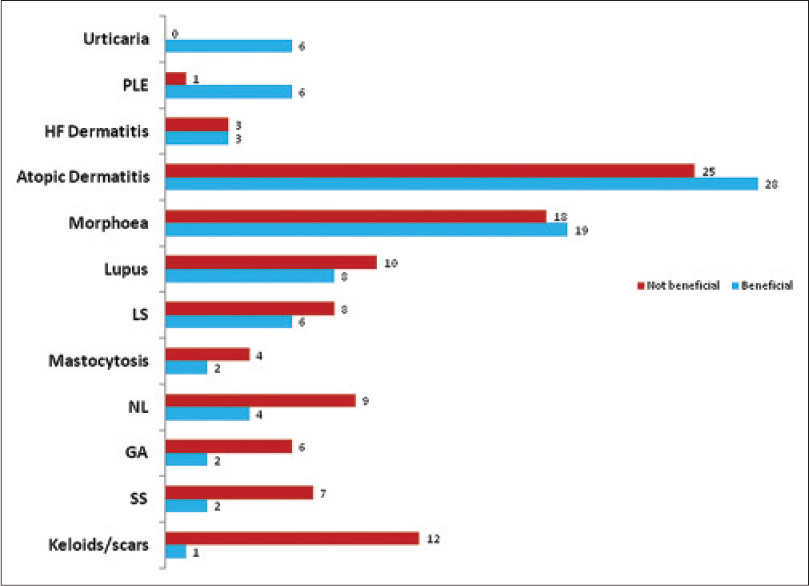 |
| Figure 1: The 12 major diseases treated with UVA1 phototherapy in descending order of “benefit”, from top to bottom. Numbers at the end of the horizontal bars indicate the number of patients in each group. GA = Granuloma annulare, HF dermatitis = Hand and foot dermatitis, NL = Necrobiosis lipoidica, SS = Systemic sclerosis |
The majority of treatment courses were administered with the metal halide units (209 of 361). Minimal erythema dose data were available for 116 of 152 treatment courses administered with the TL-10 lamps. The median minimal erythema dose (range, 0.3–20 J/cm [2]) was greater than the maximum test doses (10–20 J/cm [2]) in 66% of patients. Minimal erythema dose data from the metal halide treatment units was available for 189 of 209 treatment courses (median minimal erythema dose, 20 [range, 4–112] J/cm [2]).
Doses and treatment outcomes
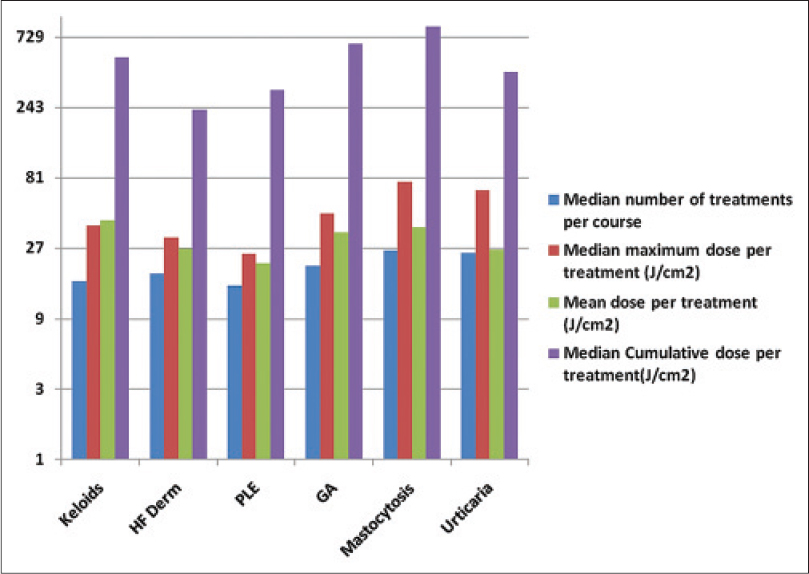
Treatment outcome data were available for only 247 of 269 patients (317 treatment courses). The majority of patients were treated with low to medium doses, though some patients were able to tolerate high doses [Figure - 2] and [Figure - 3]. Patients treated for polymorphic light eruption (desensitization) and urticaria benefited the most (6 of 7 and 6 of 6 patients, respectively). This was quite an impressive result considering that most of these patients had failed to respond to standard therapies including ultraviolet B and psoralens and ultraviolet A (PUVA).
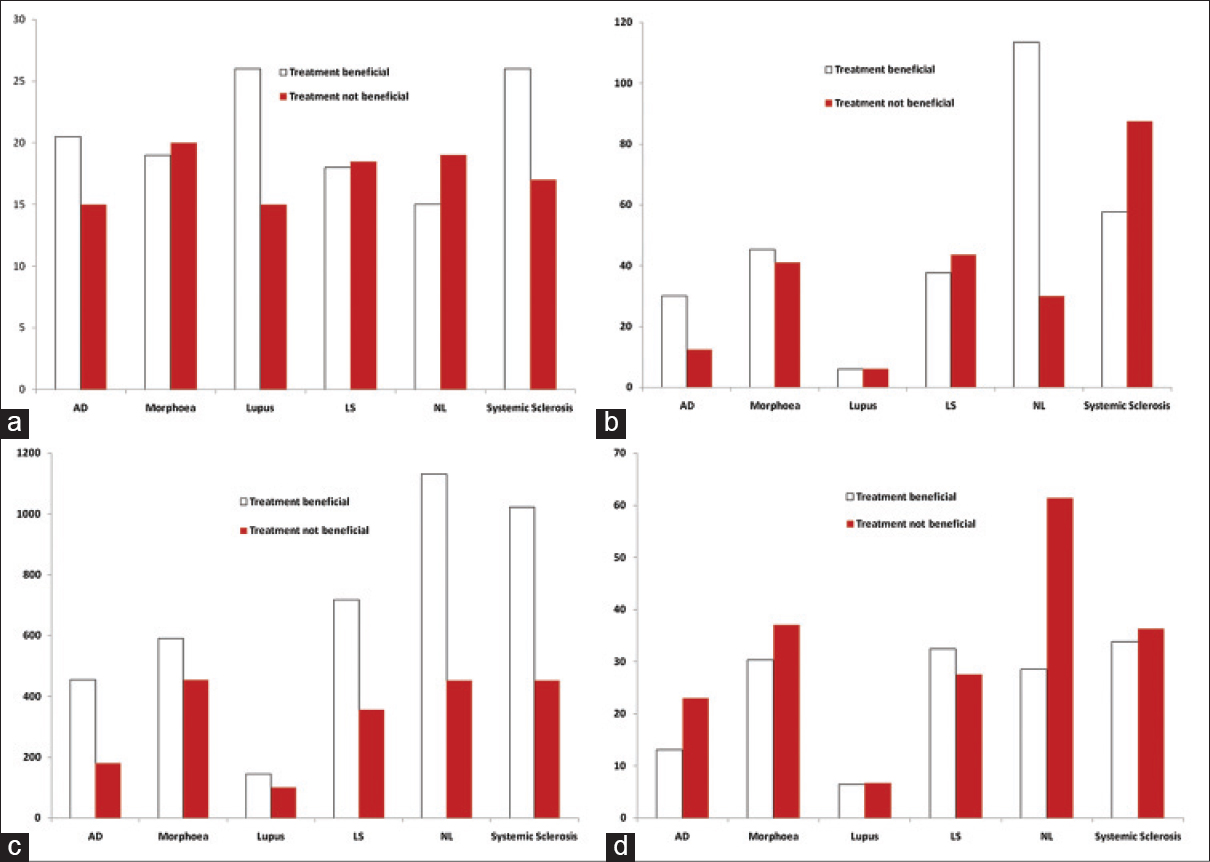 |
| Figure 2: Treatment parameters for the 6 diseases most frequently treated with UVA1 in this study. NL = Necrobiosis lipoidica. (a) Median number of treatments per treatment course. (b) Median maximum dose (J/cm[2]) per treatment course. (c) Median total dose (J/cm[2]) per treatment course. (d) Mean dose (J/cm[2]) per treatment course |
 |
| Figure 3: Treatment parameters for some of the other diseases we treated with UVA1 phototherapy. GA = Granuloma annulare, HF dermatitis = Hand and foot dermatitis |
Patients with morphea and atopic dermatitis had mixed results with just over 50% noticing “benefit.” This often meant softening of morpheic plaques and significant improvement in or complete clearance of atopic dermatitis. Unfortunately, this being a retrospective audit, reliable remission/relapse data were not available. However, akin to our experience with other phototherapy modalities for atopic dermatitis, some patients who improved after their first course of UVA1 failed to improve after their second course.
Six of 14 patients with lichen sclerosus noticed softening and repigmentation of lesional skin. No difference in response rates between genital and extragenital involvement was observed. Treatment of keloidal and hypertrophic scars was disappointing with only 1 of 13 patients noticing “definite improvement.” Similarly, only 2 of 9 patients with systemic sclerosis noticed softening of their skin and increased joint mobility. However, for the two patients who did benefit, improvement was significant and repeated courses kept their cutaneous disease under control. Though slowing down of disease progression was noted, UVA1 did not induce remission or completely reverse the fibrosing process.
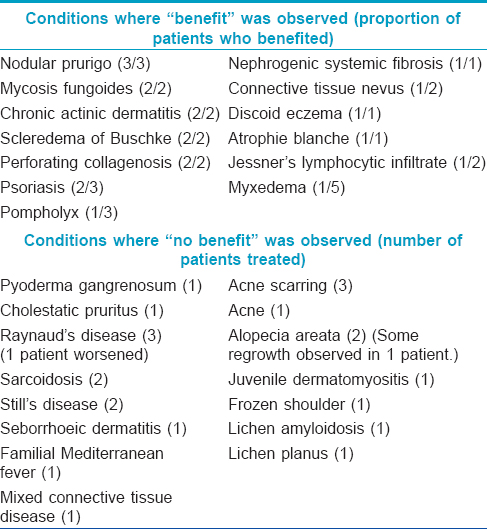
Six of 14 patients with cutaneous lupus noticed “benefit” and were able to avoid systemic immunosuppression. Lupus subtypes treated included tumid lupus, discoid lupus and subacute cutaneous lupus erythematosus (SCLE). All patients treated for subacute cutaneous lupus erythematosus noticed “benefit.” Two out of four patients with necrobiosis lipoidica who noticed “benefit” had near complete clearance of their disease. One of these patients was initially treated with the low-output TL-10 lamps (maximum dose reached = 25.8 J/cm [2]) without significant effect, but noted improvement when switched on to the metal halide high-output lamps (maximum tolerated dose = 97 J/cm [2]). Another patient had a similar experience, though the improvement was not as dramatic. [Table - 2] details other miscellaneous diseases that were treated with UVA1. Although the number of patients treated was small, all patients treated for mycosis fungoides, nodular prurigo, scleredema and perforating collagenosis noticed “benefit.” Marked improvement in patients treated for nephrogenic systemic fibrosis has been reported in some case reports.[8],[9] One patient in our series with progressive nephrogenic systemic fibrosis on chronic hemodialysis has had more than 300 treatments to date and UVA1 has been the only treatment that has slowed down the progress of his disease. He continues to undergo twice-weekly maintenance phototherapy, though UVA1 has not helped completely halt the progression of disease.
Adverse effects [Table - 3]
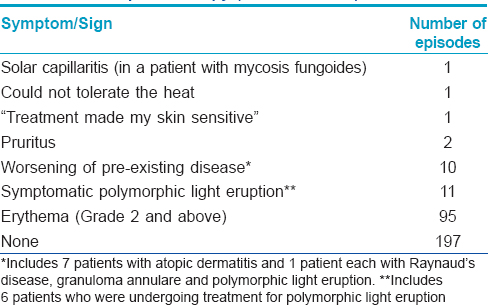
Hyperpigmentation and transient Grade 1 erythema are seen in all patients treated with UVA1 and are usually not recorded as adverse effects. Similarly, dry skin and mild pruritus are frequent and when not particularly symptomatic, go unrecorded.
Ninety five treatment courses had to be interrupted due to episodes of symptomatic Grade 2 erythema. However, all of these patients were able to continue treatment after reductions in doses and increments. No patient had to stop treatment because of erythema. However, 10 out of 269 patients had to stop treatment due to worsening of disease. Only 11 patients had documented polymorphic light eruption, of whom 6 were already being treated for the disease. However, it is possible that documentation of a common side effect such as polymorphic light eruption was not accurately performed. No malignancy was detected within the period of this study.
Limitations
The major limitation of a retrospective audit of this nature is the lack of strict objective data to assess individual outcomes including periods of remission and relapse rates across the wide range of diseases treated. Recall bias with regards to treatment benefit was probably unavoidable in cases where documentation was poor.
Discussion
The response rates we observed with UVA1 are not as impressive as those in previously published case studies and randomized trials.[2],[3],[4],[5],[10],[11] However, it should be noted that we treated patients with recalcitrant disease unresponsive to standard therapies. It is also possible that our relatively low response rates were related to higher erythemal sensitivities in our patients limiting the maximum irradiation doses. A previous study in our unit showed that the median UVA1 minimal erythema dose in the local population was 20 (range, 10–112) J/cm [2].[7]
The mean dose administered per treatment in patients with atopic dermatitis and morphea was only 20 and 35 J/cm [2] respectively. Although the median minimal erythema dose values for patients tested with the TL-10 lamps was higher than the maximum test doses (often ≤20 J/cm [2]), the median minimal erythema dose for patients tested with the metal halide lamps was 20 J/cm [2]. Most published studies describe UVA1 used at medium-high doses,[3],[5] but such a regime in our population would probably have led to a significant number of uncomfortable erythemal episodes. Though it would seem logical that the maximum tolerated dose and consequently, efficacy are related to skin phototype, a multi-center review of 92 patients treated with a range of low-high dose UVA1 dosing regimens observed a trend towards improved responses in patients of lower skin phototypes.[10] However, statistical significance was not commented upon; the relatively small numbers in that study (and ours) probably do not allow for meaningful statistical evaluation of this effect.
In the above case series, efficacy/response was graded on a 4-point scale (poor, fair, moderate and good response). Although it is difficult to directly compare results of studies with different grading systems, their results were in keeping with our data. A moderate-good response was recorded in 50% and 37.8% of patients with atopic dermatitis and morphea or lichen sclerosus, respectively. Sub-analysis of patients with morphea/lichen sclerosus showed that patients treated with medium-high doses reported higher efficacy rates (70–72.7%) than patients treated with low-dose UVA1 (46.2%). Another review of 230 patients treated with UVA1 reported efficacy rates of 84.8% and 79.6% respectively for atopic dermatitis and morphea.[3] Though their grading of efficacy was slightly different from ours, analysis of their patients who had marked improvement or clearance of disease suggests that their efficacy rates were probably similar to our patients'.
Conclusion
This review on the use of UVA1 phototherapy includes the largest number of patients reported to date. Though response rates were not very high, in the absence of safer and effective alternatives, it is worth trying UVA1 phototherapy in selected patients with recalcitrant inflammatory and sclerosing dermatoses unresponsive to traditional therapies. Long-term cutaneous malignancy risk is yet to be thoroughly evaluated. Well-designed prospective comparative studies are needed to further assess the role of UVA1 phototherapy in the management of skin diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gambichler T, Terras S, Kreuter A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: Facts and controversies. Clin Dermatol 2013;31:438-54.
[Google Scholar]
|
| 2. |
Dawe RS. Ultraviolet A1 phototherapy. Br J Dermatol 2003;148:626-37.
[Google Scholar]
|
| 3. |
Rombold S, Lobisch K, Katzer K, Grazziotin TC, Ring J, Eberlein B. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed 2008;24:19-23.
[Google Scholar]
|
| 4. |
Suh KS, Kang JS, Baek JW, Kim TK, Lee JW, Jeon YS, et al. Efficacy of ultraviolet A1 phototherapy in recalcitrant skin diseases. Ann Dermatol 2010;22:1-8.
[Google Scholar]
|
| 5. |
York NR, Jacobe HT. UVA1 phototherapy: A review of mechanism and therapeutic application. Int J Dermatol 2010;49:623-30.
[Google Scholar]
|
| 6. |
Kerr AC, Ferguson J, Attili SK, Beattie PE, Coleman AJ, Dawe RS, et al. Ultraviolet A1 phototherapy: A British photodermatology group workshop report. Clin Exp Dermatol 2012;37:219-26.
[Google Scholar]
|
| 7. |
Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. Dose-response and time-course characteristics of UV-A1 erythema. Arch Dermatol 2005;141:1549-55.
[Google Scholar]
|
| 8. |
KafiR, Fisher GJ, Quan T, Shao Y, Wang R, Voorhees JJ, et al. UV-A1 phototherapy improves nephrogenic fibrosing dermopathy. Arch Dermatol 2004;140:1322-4.
[Google Scholar]
|
| 9. |
Tran KT, Prather HB, Cockerell CJ, Jacobe H. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol 2009;145:1170-4.
[Google Scholar]
|
| 10. |
Tuchinda C, Kerr HA, Taylor CR, Jacobe H, Bergamo BM, Elmets C, et al. UVA1 phototherapy for cutaneous diseases: An experience of 92 cases in the United States. Photodermatol Photoimmunol Photomed 2006;22:247-53.
[Google Scholar]
|
| 11. |
Kreuter A, Hyun J, Stücker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol 2006;54:440-7.
[Google Scholar]
|
Fulltext Views
4,402
PDF downloads
2,938





