Translate this page into:
Update on etiopathogenesis and treatment of Acne
Corresponding Author:
Yasmeen Jabeen Bhat
Department of Dermatology, STD and Leprosy, Government Medical College, University of Kashmir, Srinagar - 190 010, Jammu and Kashmir
India
yasmeenasif76@gmail.com
| How to cite this article: Bhat YJ, Latief I, Hassan I. Update on etiopathogenesis and treatment of Acne. Indian J Dermatol Venereol Leprol 2017;83:298-306 |
Abstract
Acne, the most common skin disease, is a disorder of pilosebaceous units that affects adolescents mainly and adults occasionally. The pathogenesis is multifactorial. Besides genetic predisposition, other major factors include the action of androgens, pro-inflammatory lipids acting as ligands of peroxisome proliferator-activated receptors in the sebocytes, toll-like receptor-2 acting on keratinocytes, recognition of pathogen-associated molecular patterns, cytokines, chemokines, inflammasomes, neuroendocrine regulatory mechanisms, diet and other pro-inflammatory targets implicated in the activation of immune detection and response. Most of these factors converge on mammalian target of rapamycin complex1 (mTORC1) activation which is further enhanced by the nutrient signaling of Western diet. This multitude of pathogenic factors has led to a new armamentarium of drugs for the treatment of acne. Topical anti-androgens, insulin-like growth factor-1 inhibitors, peroxisome proliferator-activated receptor-modulators, acetylcholine inhibitors, topical retinoic acid metabolism-blocking agents, vitamin D analogues, antimicrobial peptides, interleukin-1α and interleukin-1β blockers and immunotherapy are some of the novel treatment options.Introduction
Acne is the most common skin disease with a prevalence of 35%–90% in adolescents. It peaks between the ages of 14 and the beginning of the third decade, but may persist into or develop de novo in adulthood (20% men and 35% women).[1] The disease causes significant physical and psychological morbidity. Acne can be considered as a chronic disease in view of the older and the most recent definitions of chronicity by the World Health Organization.[2]
Etiopathogenesis of Acne
The pathogenesis of acne vulgaris is multifactorial. Cytokines play an important role in the pathogenesis of acne vulgaris, together with other genetic and environmental factors. Tumor necrosis factor-α-308 gene polymorphism might be a predisposing factor for acne susceptibility with no apparent relation to its severity.[3] Acne develops as a result of an interplay between the following four factors [Figure - 1].[4],[5] In addition, research in the areas of diet and nutrition, genetics and oxidative stress have also yielded some interesting insights into the development of acne.[6]
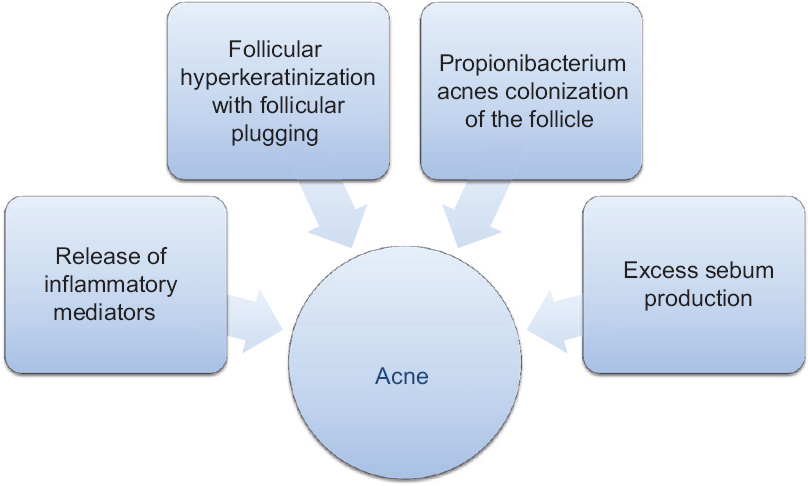 |
| Figure 1: Interplay of factors in the etiopathogenesis of acne |
Release of inflammatory mediators into the skin
Inflammation is regarded as a key component in the pathogenesis of acne.[7] An increase in the activity of the pro-inflammatory cytokine, interleukin (IL)-1, is observed before the beginning of hyperproliferation around the uninvolved follicles and is thought to trigger the activation of keratinocyte proliferation.[8] Nuclear factor kappa beta (NF- κβ) regulated mRNA gene levels of the cytokines- tumour necrosis factor (TNF)-α, IL-1 β, IL-8 and IL-10 are significantly upregulated in acne-involved skin, compared to the uninvolved normal adjacent skin. In inflammatory acne lesions, these also include many pro-inflammatory cytokine genes including those of matrix metalloproteinases, β-defensin 4, IL-8 and granulysin.[9] Elevated expression of the chemokine IL-8 and the activated protein, activator protein (AP)-1, attracts circulating inflammatory cells into the tissue. Inflammation is further characterized by the action of active lipid mediators such as leukotrienes, prostaglandins and 15-hydroxyeicosatetraenoic acids (15-HETE). These molecules are synthesized from arachidonic acid or linolenic acid by the enzyme lipoxygenase (LOX) and cyclooxygenase (COX), respectively. Both COX isozymes, COX-1 and COX-2, along with 5-LOX are expressed in human sebocytes in vitro. In particular, COX-2 expression is selectively upregulated in acne-involved sebaceous glands in vivo. Phosphodiesterases lower the intracytoplasmic levels of cAMP, leading to the preferential expression of pro-inflammatory cytokines such as TNF-α, IL-1, IL-8, IL-12 and IL-23.[10],[11] Interleukin-1 triggers remodeling of the pilosebaceous unit and promotion of comedogenesis. Interleukin-8 is important in attracting neutrophils to the site of inflammation in the pilosebaceous unit. Interleukin-12 is the major pro-inflammatory cytokine produced by monocytes in response to invading Gram-positive organisms and induces expression of anti-microbial peptides such as defensins, which have been implicated in the evolution of the acne lesion.[12] Psoriasin, a member of the S100 gene family, was shown to be highly expressed in the epidermis and the ductus seboglandularis of acne-involved skin, in contrast to uninvolved controls.[13]
Toll-like receptors
Toll-like receptors are a subtype of pattern recognition receptors (PRRs) that can activate innate immune responses through keratinocytes, neutrophils, monocytes/macrophages, natural killer cells and dendritic cells (including Langerhans cells). There are many different toll-like receptors (TLR); but TLR-2 and TLR-4 appear to be specific for acne pathogenesis. Microbial ligands (such as Propionibacterium acnes) can activate several pathways that ultimately set off nuclear factor (NF)-κβ transcription factor which causes the release of inflammatory cytokines (IL-1, IL-6, IL-8, IL-10, IL-12 and TNF-α). Toll-like receptor activation also leads to the release of antimicrobial peptides (human β defensin 1 and human β defensin 2) that play an important role in innate immune responses.[14] Toll-like receptor-mediated cytokines additionally induce matrix metalloproteinases that contribute to acne inflammation, dermal matrix destruction and scar formation.[15]
Follicular hyperkeratinization with subsequent plugging of the follicle
Toll-like receptor activation and secretion of IL-1α from keratinocytes may be the initiating steps in comedogenesis and therefore, critical to the pathophysiology of acne. Interleukin-1α is released from the infundibular keratinocyte in response to P. acnes- mediated TLR activation and is an important step in the complex natural evolution of the acne lesion.[16] Moreover, IL-1α may contribute to both the creation of a comedogenic cytokine milieu, as well as eventual sebocyte hypercornification, characteristic of acne lesions. Microcomedone, the precursor lesion in acne, results from both follicular keratinization and reduced desquamation of keratinocytes in the infundibulum, thereby forming a keratin plug at the follicular infundibulum.[17] Epithelial hyperproliferation (comedo formation) is driven by increased levels or sensitivity to androgens, changes in sebum lipid composition, P. acnes overgrowth and local cytokine mileu [Figure - 2]. Biofilm, a complex aggregation of microorganisms encased within an extracellular polysaccharide lining secreted by bacteria, has a role in the formation of a microcomedo by acting as a biological glue; de novo formation of inflammatory lesions has also been proven.[18]
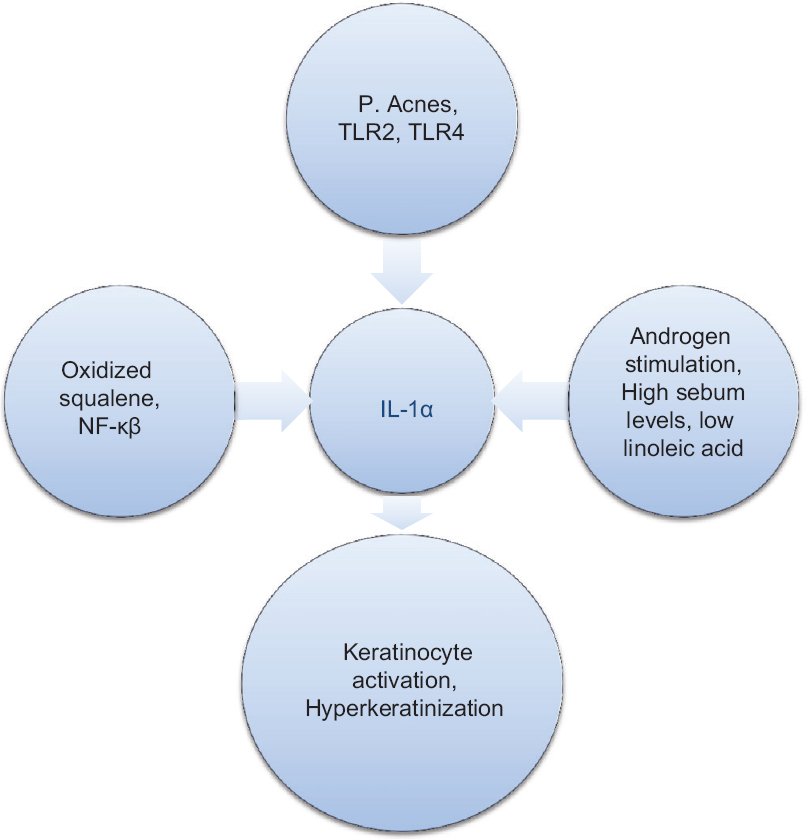 |
| Figure 2: Inflammation and comedone formation |
Propionibacterium acnes follicular colonization
P. acnes, a Gram-positive anaerobic bacteria normally found in the sebaceous follicle, plays an important role, both directly and indirectly, in the development of inflammatory acne. Other propionibacteria that may have a role include Propionibacterium granulosum and Propionibacterium avidum. P. acnes releases many enzymes such as proteinases, lipases, hyaluronidases and chemotactic factors that are integral in the inflammatory cascade.[19] It directs immune reactions by modulation of the T helper 1/T helper 2 response and induction of monocyte-derived dendritic cell maturation.[20]P. acnes stimulates the host innate immune response by activating toll-like receptors and recognizing pathogen-associated molecular patterns (PAMPs).[21],[22]
P. acnes also stimulates inflammasome formation, which are large complexes formed when PAMPs are sensed by DAMP (damage associated molecular patterns) from the host leading to the activation of caspase-1, IL-1β and IL-18 which produce the inflammatory papules of acne [Figure - 3].[23],[24]
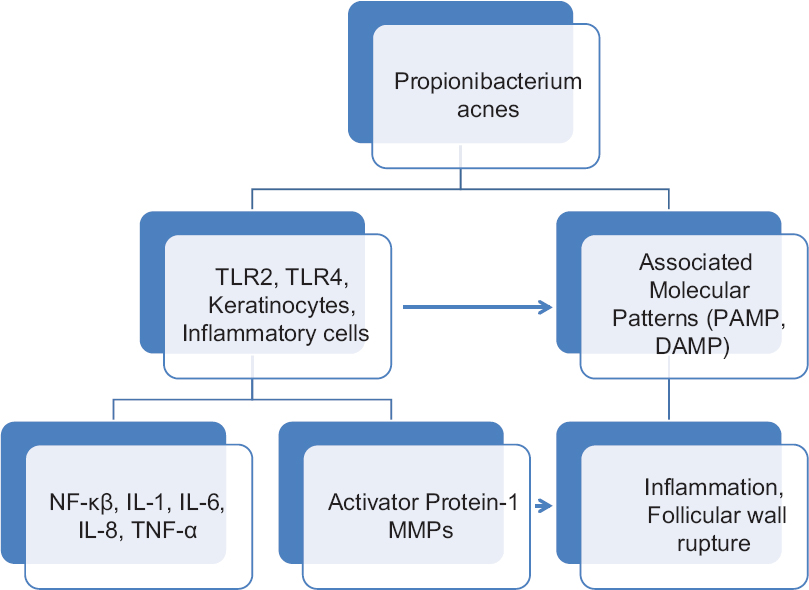 |
| Figure 3: Role of Propionibacterium acnes in the pathogenesis of acne |
Role of sebum in acne
The normal function of sebaceous glands is to produce and secrete sebum, a group of complex oils including triglycerides and fatty acid breakdown products, wax esters, squalene, cholesterol esters and cholesterol.[25] Increased sebum excretion, alteration of lipid composition and changes in the oxidant/antioxidant ratio characteristic of the skin surface lipids are the main events in acne pathogenesis.[26] The composition of lipids is of importance. Decreased levels of linoleic acid have been found in skin lipids of acne patients.[27] An important hallmark of sebum in acne patients is the presence of lipoperoxides, mainly due to the peroxidation of squalene and a decrease in the level of vitamin E, the major sebum antioxidant.[28] Both lipoperoxides and monounsaturated fatty acids (MUFA) are capable of inducing alteration in keratinocyte proliferation and differentiation, whereas peroxides are capable of inducing production of pro-inflammatory cytokines and activation of peroxisome proliferator-activated receptors (PPAR) [Figure - 4].[29]
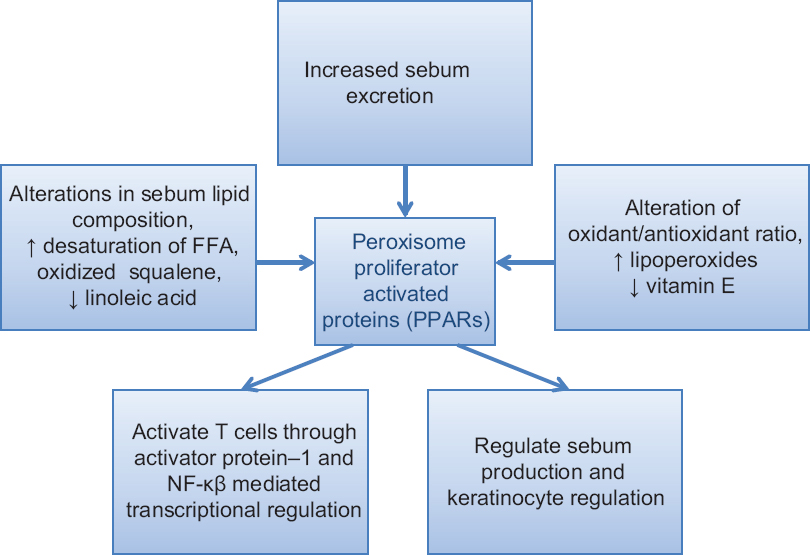 |
| Figure 4: Role of sebum in acne development |
The biological function of sebocytes is further regulated by several factors including the ligands of receptors expressed in sebocytes; such as androgens and estrogens, PPAR ligands and neuropeptides (NP), liver-X receptor ligands, histamines, retinoids and vitamin D.
Sebaceous function can also be modified by histamine and conversely, antihistamines, since histamine receptors have been identified in human sebaceous gland cells.[29] Retinoids also affect the biological function of sebocytes. Retinoic acid receptors (isotypes α and γ) and retinoid X receptors (isotypes α, β, γ) are expressed in human sebocytes. All isoforms of all transretinoic acid exhibit antiproliferative effects and inhibit sebocyte differentiation and lipid synthesis.[30],[31] Neuropeptides (with hormonal and nonhormonal actions) can also control the development of clinical inflammation in acne (neurogenic co-control). SubstancePcan be identified in numerous immune-reactive nerve fibers of acne skin and sebaceous glands respond to it with the synthesis of the neutral endopeptidase.[32]
Vitamin D receptor, vitamin D-25-hydroxylase, 25-hydroxyvitamin D-1alpha-hydroxylase and 1, 25-dihydroxyvitamin D-24-hydroxylase are expressed in SZ95 sebocytes in vitro. Furthermore, incubation of SZ95 sebocytes with 1,25 (OH)2D3 leads to a dose-dependent modulation of cell proliferation, cell cycle regulation, lipid content and IL-6/IL-8 secretion in vitro.[33]
Acne and hormones
Androgens have long been implicated in acne pathogenesis.[34] Androgens such as testosterone, dehydroepiandrosterone sulfate and dihydrotestosterone, are known to regulate genes responsible for sebaceous gland growth and sebum production.[26] The isozyme 5α-reductase type 1 which catalyzes the conversion of testosterone to 5α-dihydrotestosterone in peripheral tissues by a NADPH-dependent reaction, is expressed predominantly in skin.[35] Higher activity of type 1, 5-α reductase is seen in acne patients whereas higher levels of DHEAS is usually seen in prepubertal acne patients. Dehydroepiandrosterone sulfate has also been shown to regulate sebum production, especially in postmenopausal women, through indirect mechanisms.[29] Estrogen may exert its effects through several mechanisms: Direct opposition effect on androgens, inhibition of androgen secretion or modulation of genes involved in sebaceous gland growth and function.[34],[36] Decreased levels of estrogen in patients with acne have been found in various studies.[37] It is suggested that exogenous estrogens have a beneficial effect on acne. This is also supported by the fact that acne is most common at puberty due to the low level of estrogens during the first few menstrual cycles.[38]
A strong increase in sebum secretion occurs a few hours after birth; this peaks during the 1st week and slowly subsides thereafter. A new rise takes place at about the age of 9 years with adrenarche and continues up to the age of 17 years, when the adult level is reached.[26] Growth hormone is secreted by the pituitary and it stimulates the production of insulin-like growth factors (IGF). Sebocytes express receptors for IGF-1, the interactions resulting in the growth of the sebaceous gland.[39] Proopiomelanocortin, corticotropin-releasing hormone and corticotropin-releasing hormone receptor genes are present in the skin.[40] Corticotropin-releasing hormone (CRH) has been reported to promote lipogenesis and to enhance mRNA expression of Δ5-3-β-hydroxysteroid dehydrogenase, the enzyme that converts dehydroepiandrosterone to testosterone in human sebocytes.[41] Ganceviciene et al. hypothesized that CRH may interact with immune factors causing release of inflammatory mediators in acne.[42] Alpha-melanocyte-stimulating hormone has been implicated in increased sebogenesis in rodents via the stimulation of two receptor subtypes; the melanocortin receptor 1 and the melanocortin receptor 5, both of which are expressed in human sebocytes.[43],[44],[45]
Role of diet in acne
Acne in adolescents of developed countries is an epidemic skin disease and has currently been linked to the Western diet. High glycemic load and dairy protein consumption both increase insulin/insulin-like growth factor-1 (IGF-1) signaling that is superimposed on elevated IGF-1 signaling of puberty.[46] The cell's nutritional status is primarily sensed by the forkhead box transcription factor O1 (FoxO1) and the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1). FoxO1 links nutrient availability to mTORC1-driven processes: Increased protein and lipid synthesis, cell proliferation, cell differentiation including hyperproliferation of acroinfundibular keratinocytes, sebaceous gland hyperplasia, increased sebaceous lipogenesis, insulin resistance and increased body mass index.[47],[48] Enhanced androgen levels and increased TNF-α and IGF-1 signaling due to genetic polymorphisms promoting the risk of acne, all converge in mTORC1 activation which is further enhanced by nutrient signaling of Western diet.[49] Occurrence of acne as part of various syndromes also provides evidence in favor of correlation between IGF-1 and acne.[50] Acne is absent in populations consuming palaeolithic diets with low glycemic load and no consumption of milk or dairy products.[51]
Recent progress in understanding the nutrient-sensitive kinase mTORC1 allows a new view of nutrient signaling in acne by both high glycemic load diet and increased insulin, IGF-1 and leucine signaling due to milk protein consumption. Acne should be regarded as an mTORC1-driven disease of civilization, such as obesity, type 2 diabetes and cancer induced by Western diet [Figure - 5].[49]
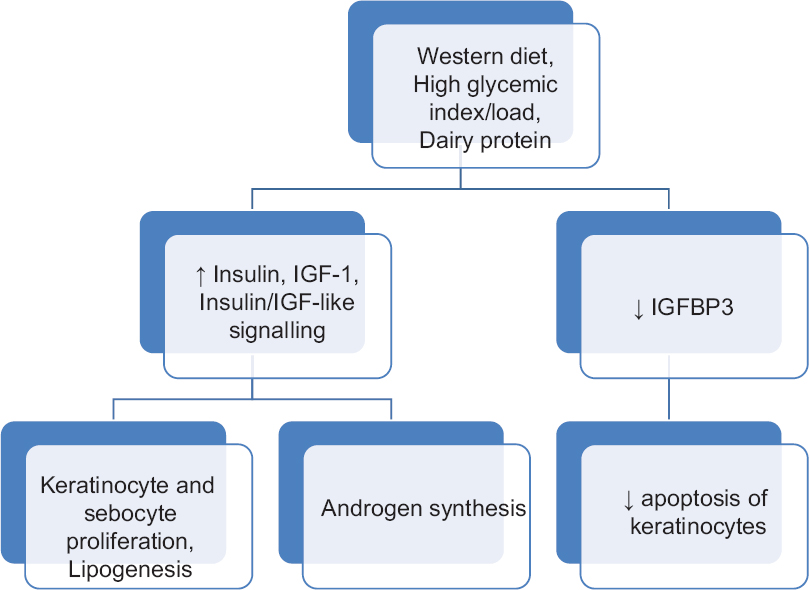 |
| Figure 5: Role of diet in acne |
Clinical Features and Assessment
Acne affects the areas of skin with the densest population of sebaceous follicles; the prevalence and severity on the face, chest and back being 92%, 45% and 61%, respectively.[52] In particular, the number of acne patients outside the classic age range, as in acne tarda, is increasing.[53]
Familial predisposition, especially acne in the mother, is significantly associated with a more severe course.[54] Exogenous factors such as androgenic hormones, competitive sports, nicotine and diet with a high hyperglycemic index make the natural course of acne more severe and persistent.[55] In the Glasgow alumni cohort study, students with and without a history of acne were compared; a positive acne history correlated with a clearly reduced risk of coronary artery disease but a higher risk for prostate cancer.[56]
Acne vulgaris is the common type, prevalent in 99% of the acne cases, the lesions being comedones, papules, pustules, nodules, cysts and associated scarring. The other types of acne are acne conglobata, acne excoriee, acne rosacea, acne cosmetica, mallorca acne, pomade acne, acne fulminans, acne keloidalis nuchae, chloracne, acne mechanica and acne medicamentosa.
Acne assessment
Till now, there are more than 25 different grading systems for the assessment of acne severity that have been published in literature. Lehmann et al. have surveyed at least 25 scales for assessing the global severity of acne.[57] Generally, a grading system aims to achieve simplicity, accuracy and a quick assessment. However, the existence of so many grading systems indicates a lack of consensus on this issue and hence no grading system is considered to be a global standard.[58],[59],[60],[61],[62]
Computational methods for assessment of acne lesions
Researchers have proposed computational imaging methods for aiding in the acne clinical severity grading. Phillips et al. were the first ones to study polarized light photography to assess the comedo counts and inflammatory acne lesion counts.[63] This enhanced the visualization of skin features, color and lighting and were framed in perpendicular polarized photographs.
In 2001, Rizova and Kligman used both parallel and cross polarizing light photography in combination with video microscopy and sebum production measurement.[64] In 2008, Do et al. studied the computer assisted alignment and tracking of acne.[65] Another group of researchers exploited multispectral images, capturing image data at specific wavelengths across the electromagnetic spectrum.[66]
Novel Treatment of Acne
The current effective strategies of management recommended by the global alliance are directed toward one or more of these pathogenic factors and include topical and systemic antibiotics and retinoids, benzoyl peroxide, azelaic acid, salicylic acid and oral antiandrogens, depending on the severity of the disease. However, because of unwanted side effects (irritation, bacterial resistance, systemic side effects) and chronicity, new treatments that target the different pathogenic mechanisms of acne with minimal side effects are desirable.[4] The risk of rhabdomyolysis with isotretinoin has been known for 30 years and is small, but every patient should be warned not to engage in over-strenuous activity at work or sports.[67] Clinical observation and laboratory monitoring are recommended. Further research is directed at targeting receptors, adhesion molecules, cytokines, chemokines or other pro-inflammatory targets implicated in the activation of immune detection and response (i.e., TLRs, PPARs) that appear to contribute to the pathophysiology of acne.[68],[69] Therapeutic options that reduce the need for topical and/or oral antibiotic therapy for acne are welcome as bacterial resistance to antibiotics is a clinically relevant concern.[70]
Agents that primarily target sebum production
Topical antiandrogens
Due to their oral administration and consequent systemic side effects, antiandrogens are only recommended in the treatment of moderate-to-severe acne in female patients who have not responded to conventional therapy alone.[34],[71] Antiandrogens for topical use are currently undergoing clinical trials and seem to offer a safe approach for controlling sebogenesis.
Cortexolone 17α-propionate (CB-03-01)
Cortexolone 17α-propionate, also known as CB-03-01, is a new topical monoester of cortexolone which possesses more potent anti-androgenic actions than other antiandrogens without any systemic side effects.[72] An interaction with the androgen receptor is proposed as the mechanism of action.[73]
ASC-J9 cream
ASC-J9 selectively promotes the degradation of the androgen receptor and thus exerts its antiandrogenic effects by inhibiting the interaction of circulating androgens with their receptor.[74] A topical formulation of ASC-J9, ASC-J9 cream has been shown to reduce the sebaceous gland size and decrease sebum production.[75] No systemic or local side effects were reported, thus making ASC-J9, a drug with an adequate safety profile.[76]
NVN1000
NVN1000, also called SB204, is a gel that releases nitric oxide when applied topically to the skin.[77] By inhibiting cytochrome 450 and reducing 5-α reductase activity, both of which can be found in sebocytes and are required for independent skin steroidogenesis, nitric oxide is able to decrease skin androgen levels, consequently reducing sebocyte proliferation, and sebum production.[78],[79],[80] Further, as a reactive free radical, nitric oxide exhibits antibacterial effects.[81] In humans, the tolerability, safety and efficacy of different concentrations (1%, 4%, 8%) of NVN1000 have also been evaluated.[82],[83]
Melanocortin receptor antagonists
JNJ 10229570, a melanocortin receptor 1 and melanocortin receptor 5 antagonist, decreases the size of sebaceous glands, production of sebaceous lipids and the expression of the sebaceous differentiation marker epithelial-membrane antigen in cultured primary human sebocytes.[84]
Insulin-like growth factor-1 inhibitors
Epigallocatechin-3-gallate (EGCG), a major polyphenolic constituent in green tea, significantly reduces size of sebaceous glands, the mean number of sebocytes per gland and the size of comedones, inhibits cell proliferation and lipid synthesis in SZ95 sebocytes in vitro via inhibiting IGF-1.[85] EGCG has also shown to inhibit 5α-reductase-1 activity, and thus limit dihydrotestosterone-dependent sebum production.[86] Further, EGCG exerts antimicrobial activity against P. acnes and has proven to be effective in treatment of acne.[87] Patients treated with EGCG solution showed a 79%–89% reduction in non-inflammatory and inflammatory lesion counts after 8 weeks of treatment. Zinc gluconate has also been shown to reduce the over expression of IGF-1 and IGF-1 receptor caused by P. acnes.[88]
Peroxisome proliferator-activated receptor modulators
Zileuton, an oral 5-lipoxygenase inhibitor, has been shown to reduce the number of inflammatory lesions in moderate acne by downregulating IL-6 and leukotriene B4 (LTB4), a ligand for PPAR-α, and by temporarily inhibiting the synthesis of sebaceous lipids.[89]
Acetylcholine inhibitors
Sebaceous glands express acetylcholine receptors in a highly regulated manner, suggesting a role of acetylcholine in sebum production, probably through promoting sebocyte differentiation.[90] Botulinum toxin inhibits the presynaptic release of acetylcholine and was recently found to noticeably decrease sebum production, oiliness of skin and pore size.[91] Topical anticholinergic agents (poldine methylmethosulfate) have also shown to reduce sebum production.[92]
Acetyl coenzyme A carboxylase (ACC) inhibitors
Acetyl coenzyme A carboxylase catalyzes the conversion of acetyl-coenzyme A into malonyl-coenzyme A which in turn has a role in determining whether fatty acids are synthesized or oxidized. Inhibition of ACC has shown to significantly increase fatty acid oxidation and to reduce triglyceride synthesis.[93] DRM01B (an inhibitor of the enzyme ACC) 7.5% gel is under trial.[94]
Agents that primarily normalize abnormal keratinization within the pilosebaceous unit
Retinoic acid metabolism-blocking agents
Talarozole
Is a selective azole derivative that potently inhibits the cytochrome CYP26, an isozyme involved in the metabolism of retinoic acid (RA).[95] The rationale is that talarozole inhibits cytochrome CYP26 and increases the level of retinoic acid, allowing for normalization of desquamation of the follicular epithelium and thus reducing comedo formation.[96] A gel formulation containing 0.35 and 0.7% talarozole provides the effects of retinoic acid formulations with less irritation.[97]
Monoclonal antibodies anti interleukin-1α
Interleukin-1α seems to play a role in comedo formation. P. acnes activates the release of IL-1α via TLR-2 activation.[98] Retinoic acid RA-18C3, a monoclonal antibody specific for IL-1α, is used to treat patients with moderate-to-severe acne. Subcutaneous injections of 100 or 200 mg of RA-18C3 is given on days 0, 21 and 42 for a total of three injections shows significant improvement.[99]
Agents that primarily work by modulating Propionibacterium acnes
Antimicrobial peptides
MBI 226, currently known as omiganan pentahydrochloride, is a topical cationic peptide derived from the bovine antimicrobial peptide indolicidin that is shown to have rapid (2–6 h) in vitro microbicidal activity against a variety of Gram-positive and Gram-negative bacteria by disrupting their cytoplasmic membranes and causing depolarization followed by cell death.[100],[101] MBI 226 2.5% and 5.0% solutions when used topically in the treatment of acne vulgaris for 6 weeks, reduced the count of comedones, papules and pustules.[102]
Antioxidants
Vitamin C, a potent antioxidant and reactive oxygen species scavenger, has shown to exert antimicrobial effects on P. acnes, prevent up to 40% of ultraviolet A-induced sebum oxidation and improve acne lesions in up to 76.9% of patients.[103],[104],[105]
Agents that primarily work by modulating the inflammatory response
Phosphodiesterase inhibitors
Because phosphodiesterase 4 is the main cAMP-degrading isoenzyme, its inhibition elevates cyclic adenosine monophosphate levels and thus decreases the activity of pro-inflammatory cytokines. Therefore, drugs such as apremilast, a small-molecule phosphodiesterase 4 inhibitor, could potentially play a role in acne treatment in the future.[106]
Inhibitors of interleukin-1β-mediated inflammatory response
Gevokizumab, also known as XOMA 052, is a humanized monoclonal immunoglobulin G2 antibody that shows high affinity and specificity to IL-1β which plays a role in inflammatory acne.[107],[108] The potential use of gevokizumab to treat moderate to severe acne vulgaris is currently being studied.
Vitamin D analogues
Treatment of cultured sebocytes with vitamin D decreases the expression of IL-6, IL-8 and matrix metalloproteinase-9.[109] Further, vitamin D and its analog calcipotriol are shown to regulate innate immunity by inducing the expression of antimicrobial peptides, such as β-defensin and cathelicidin LL-37, in cultured keratinocytes.[110],[111] The efficacy of 1 gram calcipotriene cream applied twice daily in the treatment of acne is currently being studied.
Dapsone 5%
A topical formulation of dapsone has been approved by the FDA for the treatment of acne vulgaris. Data suggests that dapsone gel (5%) due to its antibacterial and anti-inflammatory effects has the potential to become an established topical drug for the treatment of acne vulgaris.[112]
Systemic antiandrogens
Antiacne effects of oral contraceptives is due to the decreasing levels of circulatory androgens through inhibition of luteinizing hormones and follicle stimulating hormone.[113],[114] The currently FDA approved agents include norgestimate with ethinyl estradiol and norethindrone acetate with ethinyl estradiol. Cyproterone acetate is an androgen receptor blocking agent which has been well studied and found to be effective in acne in females. Higher doses have been found to be more effective than lower dose. It is also combined (2 mg) with ethinyl estradiol (35 or 50 μg) as an oral contraceptive formulation to treat acne. Other androgen blockers used in acne are spironolactone, flutamide and finasteride.[115]
Immunotherapy
The role of P. acnes in acne confers legitimacy on the possible benefits of immunization-based approaches which may represent a solution for limiting the development of antibiotic-resistant P. acnes. Various immunization-based approaches have been developed in the last decades, including killed pathogen-based vaccines, vaccination against cell wall-anchored sialidase, monoclonal antibodies to the Christie, Atkins, Munch-Peterson factor of P. acnes, anti-toll-like receptor vaccines and natural antimicrobial peptides.[116]
Conclusion
Various cytokines, chemokines, toll-like receptors and inflammasomes are involved in the pathogenesis of acne which should be regarded as a mammalian target of rapamycin complex 1-driven disease induced by Western diet. Topical retinoic acid metabolism-blocking agents, topical antiandrogens, vitamin D receptor analogues, antimicrobial peptides, IL-1β blockers and immunotherapy are the recent treatment modalities which will soon be incorporated into newer treatment guidelines so as to target the most pathogenic factors for optimum disease control.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. | Collier CN, Harper JC, Cafardi JA, Cantrell WC, Wang W, Foster KW, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 2008;58:56-9. [Google Scholar] |
| 2. | Gollnick HP, Finlay AY, Shear N; Global Alliance to Improve Outcomes in Acne. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol 2008;9:279-84. [Google Scholar] |
| 3. | Al-Shobaili HA, Salem TA, Alzolibani AA, Robaee AA, Settin AA. Tumor necrosis factor-a -308 G/A and interleukin 10 -1082 A/G gene polymorphisms in patients with acne vulgaris. J Dermatol Sci 2012;68:52-5. [Google Scholar] |
| 4. | Thiboutot D, Gollnick H, Bettoli V, Dré no B, Kang S, Leyden JJ, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol 2009;60 5 Suppl:S1-50. [Google Scholar] |
| 5. | Harvey A, Huynh TT. Inflammation and acne: Putting the pieces together. J Drugs Dermatol 2014;13:459-63. [Google Scholar] |
| 6. | Suh DH, Kwon HH. What's new in the physiopathology of acne? Br J Dermatol 2015;172 Suppl 1:13-9. [Google Scholar] |
| 7. | Zouboulis CC. Is acne vulgaris a genuine inflammatory disease? Dermatology 2001;203:277-9. [Google Scholar] |
| 8. | Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol 2003;121:20-7. [Google Scholar] |
| 9. | Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol 2005;166:1691-9. [Google Scholar] |
| 10. | Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl) 2006;84:75-87. [Google Scholar] |
| 11. | Zouboulis CC, Seltmann H, Alestas T. Zileuton prevents the activation of the leukotriene pathway and reduces sebaceous lipogenesis. Exp Dermatol 2010;19:148-50. [Google Scholar] |
| 12. | Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol 2002;169:1535-41. [Google Scholar] |
| 13. | Ganceviciene R, Fimmel S, Glass E, Zouboulis CC. Psoriasin and follicular hyperkeratinization in acne comedones. Dermatology 2006;213:270-2. [Google Scholar] |
| 14. | McInturff JE, Kim J. The role of toll-like receptors in the pathophysiology of acne. Semin Cutan Med Surg 2005;24:73-8. [Google Scholar] |
| 15. | Selway JL, Kurczab T, Kealey T, Langlands K. Toll-like receptor 2 activation and comedogenesis: Implications for the pathogenesis of acne. BMC Dermatol 2013;13:10. [Google Scholar] |
| 16. | Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect Immun 1995;63:3158-65. [Google Scholar] |
| 17. | Thiboutot DM. Overview of acne and its treatment. Cutis 2008;81 1 Suppl:3-7. [Google Scholar] |
| 18. | Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol 2007;57:722-4. [Google Scholar] |
| 19. | Sahdo B, Särndahl E, Elgh F, Söderquist B. Propionibacterium acnes activates caspase-1 in human neutrophils. APMIS 2013;121:652-63. [Google Scholar] |
| 20. | Nakatsuji T, Rasochova L, Huang CM. Vaccine therapy for P. acnes-associated diseases. Infect Disord Drug Targets 2008;8:160-5. [Google Scholar] |
| 21. | Michalak-Stoma A, Tabarkiewicz J, Olender A, Juszkiewicz-Borowiec M, Stoma F, Pietrzak A, et al. The effect of Propionibacterium acnes on maturation of dendritic cells derived from acne patients' peripherial blood mononuclear cells. Folia Histochem Cytobiol 2008;46:535-9. [Google Scholar] |
| 22. | Jalian HR, Liu PT, Kanchanapoomi M, Phan JN, Legaspi AJ, Kim J. All-trans retinoic acid shifts Propionibacterium acnes-induced matrix degradation expression profile toward matrix preservation in human monocytes. J Invest Dermatol 2008;128:2777-82. [Google Scholar] |
| 23. | Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol 2014;134:381-8. [Google Scholar] |
| 24. | Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep 2010;10:229-35. [Google Scholar] |
| 25. | Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. Skin lipids: An update. J Invest Dermatol 1987;88 3 Suppl:2s-6s. [Google Scholar] |
| 26. | Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol 2004;22:360-6. [Google Scholar] |
| 27. | Downing DT, Stewart ME, Wertz PW, Strauss JS. Essential fatty acids and acne. J Am Acad Dermatol 1986;14(2 Pt 1):221-5. [Google Scholar] |
| 28. | Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis CC, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: A possible role in acne vulgaris. J Invest Dermatol 2006;126:2430-7. [Google Scholar] |
| 29. | Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A 2000;97:4279-84. [Google Scholar] |
| 30. | Tsukada M, Schröder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol 2000;115:321-7. [Google Scholar] |
| 31. | Zouboulis CC, Korge B, Akamatsu H, Xia LQ, Schiller S, Gollnick H, et al. Effects of 13-cis-retinoic acid, all-trans-retinoic acid, and acitretin on the proliferation, lipid synthesis and keratin expression of cultured human sebocytes in vitro. J Invest Dermatol 1991;96:792-7. [Google Scholar] |
| 32. | Thielitz A, Gollnick H. Overview of new therapeutic developments for acne. Expert Rev Dermatol 2009;4:55-65. [Google Scholar] |
| 33. | Krämer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the Vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol 2009;113:9-16. [Google Scholar] |
| 34. | George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg 2008;27:188-96. [Google Scholar] |
| 35. | Chen W, Zouboulis CC, Fritsch M, Kodelja V, Orfanos CE. Heterogeneity and quantitative differences of type 1 5 alpha-reductase expression in cultured skin epithelial cells. Dermatology 1998;196:51-2. [Google Scholar] |
| 36. | Thiboutot D. Hormones and acne: Pathophysiology, clinical evaluation, and therapies. Semin Cutan Med Surg 2001;20:144-53. [Google Scholar] |
| 37. | Russell JJ. Topical therapy for acne. Am Fam Physician 2000;61:357-66. [Google Scholar] |
| 38. | Thiboutot D, Sivarajah A, Gilliland K, Cong Z, Clawson G. The melanocortin 5 receptor is expressed in human sebaceous glands and rat preputial cells. J Invest Dermatol 2000;115:614-9. [Google Scholar] |
| 39. | Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology 2003;206:57-67. [Google Scholar] |
| 40. | Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett 1995;374:113-6. [Google Scholar] |
| 41. | Zouboulis CC, Seltmann H, Hiroi N, Chen W, Young M, Oeff M, et al. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A 2002;99:7148-53. [Google Scholar] |
| 42. | Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol 2009;160:345-52. [Google Scholar] |
| 43. | Thody AJ, Shuster S. Control of sebaceous gland function in the rat by alpha-melanocyte-stimulating hormone. J Endocrinol 1975;64:503-10. [Google Scholar] |
| 44. | Böhm M, Schiller M, Ständer S, Seltmann H, Li Z, Brzoska T, et al. Evidence for expression of melanocortin-1 receptor in human sebocytes in vitro and in situ. J Invest Dermatol 2002;118:533-9. [Google Scholar] |
| 45. | Eisinger M, Li WH, Anthonavage M, Pappas A, Zhang L, Rossetti D, et al. A melanocortin receptor 1 and 5 antagonist inhibits sebaceous gland differentiation and the production of sebum-specific lipids. J Dermatol Sci 2011;63:23-32. [Google Scholar] |
| 46. | Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol 2009;18:833-41. [Google Scholar] |
| 47. | Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol 2008;128:1286-93. [Google Scholar] |
| 48. | Sadagurski M, Yakar S, Weingarten G, Holzenberger M, Rhodes CJ, Breitkreutz D, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol 2006;26:2675-87. [Google Scholar] |
| 49. | Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol 2013;22:311-5. [Google Scholar] |
| 50. | Kumari R, Thappa DM. Role of insulin resistance and diet in acne. Indian J Dermatol Venereol Leprol 2013;79:291-9. [Google Scholar] |
| 51. | Melnik BC. Diet in acne: Further evidence for the role of nutrient signalling in acne pathogenesis. Acta Derm Venereol 2012;92:228-31. [Google Scholar] |
| 52. | Tan JK, Tang J, Fung K, Gupta AK, Thomas DR, Sapra S, et al. Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol 2008;7:551-6. [Google Scholar] |
| 53. | Zouboulis CC. Acne vulgaris. The role of hormones. Hautarzt 2010;61:107-8. [Google Scholar] |
| 54. | Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity, and severity risk factors of acne in high school pupils: A community-based study. J Invest Dermatol 2009;129:2136-41. [Google Scholar] |
| 55. | Schäfer T, Kahl C, Rzany B. Epidemiology of acne. J Dtsch Dermatol Ges 2010;8 Suppl 1:S4-6. [Google Scholar] |
| 56. | Galobardes B, Davey Smith G, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: Lower coronary heart disease but higher prostate cancer mortality: The Glasgow Alumni Cohort Study. Am J Epidemiol 2005;161:1094-101. [Google Scholar] |
| 57. | Lehmann HP, Robinson KA, Andrews JS, Holloway V, Goodman SN. Acne therapy: A methodologic review. J Am Acad Dermatol 2002;47:231-40. [Google Scholar] |
| 58. | Witkowski JA, Parish LC. The assessment of acne: An evaluation of grading and lesion counting in the measurement of acne. Clin Dermatol 2004;22:394-7. [Google Scholar] |
| 59. | Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol 2009;75:323-6. [Google Scholar] |
| 60. | Cook CH, Centner RL, Michaels SE. An acne grading method using photographic standards. Arch Dermatol 1979;115:571-5. [Google Scholar] |
| 61. | Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol 1997;36:416-8. [Google Scholar] |
| 62. | Hayashi N, Akamatsu H, Kawashima M; Acne Study Group. Establishment of grading criteria for acne severity. J Dermatol 2008;35:255-60. [Google Scholar] |
| 63. | Phillips SB, Kollias N, Gillies R, Muccini JA, Drake LA. Polarized light photography enhances visualization of inflammatory lesions of acne vulgaris. J Am Acad Dermatol 1997;37:948-52. [Google Scholar] |
| 64. | Rizova E, Kligman A. New photographic techniques for clinical evaluation of acne. J Eur Acad Dermatol Venereol 2001;15 Suppl 3:13-8. [Google Scholar] |
| 65. | Do TT, Zarkhin S, Orringer JS, Nemeth S, Hamilton T, Sachs D, et al. Computer-assisted alignment and tracking of acne lesions indicate that most inflammatory lesions arise from comedones and de novo. J Am Acad Dermatol 2008;58:603-8. [Google Scholar] |
| 66. | Masahiro N, Tsumura N, Miyake Y. Why multispectral imaging in medicine. J Imaging Sci Technol 2004;48:125-9. [Google Scholar] |
| 67. | Dalal A, Ben-Barak S, Zlotogorski A, Constantini N. Isotretinoin and exercise: Can the two walk together? Harefuah 2014;153:104-8, 125. [Google Scholar] |
| 68. | Jarrousse V, Castex-Rizzi N, Khammari A, Charveron M, Dré no B. Zinc salts inhibit in vitro Toll-like receptor 2 surface expression by keratinocytes. Eur J Dermatol 2007;17:492-6. [Google Scholar] |
| 69. | Tenaud I, Khammari A, Dreno B.In vitro modulation of TLR-2, CD1d and IL-10 by adapalene on normal human skin and acne inflammatory lesions. Exp Dermatol 2007;16:500-6. [Google Scholar] |
| 70. | Eichenfield LF, Del Rosso JQ, Mancini AJ, Cook-Bolden F, Stein Gold L, Desai S, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol 2015;14:263-72. [Google Scholar] |
| 71. | Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res 2012;304:499-510. [Google Scholar] |
| 72. | Trifu V, Tiplica GS, Naumescu E, Zalupca L, Moro L, Celasco G. Cortexolone 17a-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol 2011;165:177-83. [Google Scholar] |
| 73. | Celasco G, Moro L, Bozzella R, Ferraboschi P, Bartorelli L, Quattrocchi C, et al. Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist. Arzneimittelforschung 2004;54:881-6. [Google Scholar] |
| 74. | Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis 2013;4:e764. [Google Scholar] |
| 75. | A Phase 2 Study of ASC-J9 Cream in Acne Vulgaris. Identifier NCT00525499. Clinical Trials: A Service of the U.S National Institutes of Health. Available from: http://www.clinicaltrials.gov. [Last accessed on 2016 Jan 02]. [Google Scholar] |
| 76. | AndroScience Corp. AndroScience Corporation Announces Phase 2a Study Results on ASC-J9. For the Treatment of Acne. Available from: http://www.androscience.com/artman/publish/news. [Last accessed on 2016 Jan 02]. [Google Scholar] |
| 77. | Kelce W. Topical Nitric Oxide: A First Class Local Antiandrogen Therapy. (White Paper). Novan Therapeutics. Available from: http://www.novantherapeutics.com. [Last accessed on 2016 Jan 04] [Google Scholar] |
| 78. | Morgan ET, Ullrich V, Daiber A, Schmidt P, Takaya N, Shoun H, et al. Cytochromes P450 and flavin monooxygenases – Targets and sources of nitric oxide. Drug Metab Dispos 2001;29:1366-76. [Google Scholar] |
| 79. | Drewett JG, Adams-Hays RL, Ho BY, Hegge DJ. Nitric oxide potently inhibits the rate-limiting enzymatic step in steroidogenesis. Mol Cell Endocrinol 2002;194:39-50. [Google Scholar] |
| 80. | Fritsch M, Orfanos CE, Zouboulis CC. Sebocytes are the key regulators of androgen homeostasis in human skin. J Invest Dermatol 2001;116:793-800. [Google Scholar] |
| 81. | Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012;13:3343-54. [Google Scholar] |
| 82. | Study of NVN1000 Topical Gel and Topical Gel Vehicle in the Treatment of Moderate to Severe Acne Vulgaris: Identifier NCT01556698. Clinical Trials: A Service of the U.S National Institutes of Health. Available from: http://www.clinicaltrials.gov. [Last accessed on 2016 Jan 04] [Google Scholar] |
| 83. | A Phase 1, 3 Day Study of Safety and Tolerability of NVN1000 Topical Gel in Healthy Volunteers: Identifier NCT01755247. Clinical Trials: A Service of the U.S National Institutes of Health. Available from: http://www.clinicaltrials.gov. [Last accessed on 2016 Jan 04] [Google Scholar] |
| 84. | Zhang L, Li WH, Anthonavage M, Pappas A, Rossetti D, Cavender D, et al. Melanocortin-5 receptor and sebogenesis. Eur J Pharmacol 2011;660:202-6. [Google Scholar] |
| 85. | Im M, Kim SY, Sohn KC, Choi DK, Lee Y, Seo YJ, et al. Epigallocatechin-3-gallate suppresses IGF-I-induced lipogenesis and cytokine expression in SZ95 sebocytes. J Invest Dermatol 2012;132:2700-8. [Google Scholar] |
| 86. | Liao S. The medicinal action of androgens and green tea epigallocatechin gallate. Hong Kong Med J 2001;7:369-74. [Google Scholar] |
| 87. | Elsaie ML, Abdelhamid MF, Elsaaiee LT, Emam HM. The efficacy of topical 2% green tea lotion in mild-to-moderate acne vulgaris. J Drugs Dermatol 2009;8:358-64. [Google Scholar] |
| 88. | Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol 2008;159:990-1. [Google Scholar] |
| 89. | Zouboulis CC, Nestoris S, Adler YD, Orth M, Orfanos CE, Picardo M, et al. A new concept for acne therapy: A pilot study with zileuton, an oral 5-lipoxygenase inhibitor. Arch Dermatol 2003;139:668-70. [Google Scholar] |
| 90. | Kurzen H, Schallreuter KU. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp Dermatol 2004;13 Suppl 4:27-30. [Google Scholar] |
| 91. | Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg 2013;39(3 Pt 1):443-8. [Google Scholar] |
| 92. | Cartlidge M, Burton JL, Shuster S. The effect of prolonged topical application of an anticholinergic agent on the sebaceous glands. Br J Dermatol 1972;86:61-3. [Google Scholar] |
| 93. | Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010;45:199-214. [Google Scholar] |
| 94. | Thomson BioWorld. With $42M Series A, Dermira Amasses Dermatology Assets; 2011. Available from: http://www.pharmacychoice.com/News/article.cfm. [Last accessed on 2016 Jan 02] [Google Scholar] |
| 95. | Geria AN, Scheinfeld NS. Talarozole, a selective inhibitor of P450-mediated all-trans retinoic acid for the treatment of psoriasis and acne. Curr Opin Investig Drugs 2008;9:1228-37. [Google Scholar] |
| 96. | Stoppie P, Borgers M, Borghgraef P, Dillen L, Goossens J, Sanz G, et al. R115866 inhibits all-trans-retinoic acid metabolism and exerts retinoidal effects in rodents. J Pharmacol Exp Ther 2000;293:304-12. [Google Scholar] |
| 97. | Pavez Loriè E, Cools M, Borgers M, Wouters L, Shroot B, Hagforsen E, et al. Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. Br J Dermatol 2009;160:26-36. [Google Scholar] |
| 98. | Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol 2004;150:421-8. [Google Scholar] |
| 99. | XBiotech, Inc. “Treatment of Inflammatory Skin Disease and Psychiatric Conditions”. United States Patent US 2013/0039921 A1. Available from: https://www.google.com/patents. [Last accessed on 2016 Jan 02] [Google Scholar] |
| 100. | Sader HS, Fedler KA, Rennie RP, Stevens S, Jones RN. Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: Spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob Agents Chemother 2004;48:3112-8. [Google Scholar] |
| 101. | Faccone D, Veliz O, Corso A, Noguera M, Martínez M, Payes C, et al. Antimicrobial activity of de novo designed cationic peptides against multi-resistant clinical isolates. Eur J Med Chem 2014;71:31-5. [Google Scholar] |
| 102. | Anderegg TR, Fritsche TR, Jones RN; Quality Control Working Group. Quality control guidelines for MIC susceptibility testing of omiganan pentahydrochloride (MBI 226), a novel antimicrobial peptide. J Clin Microbiol 2004;42:1386-7. [Google Scholar] |
| 103. | Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: An overview. Indian J Clin Biochem 2013;28:314-28. [Google Scholar] |
| 104. | Klock J, Ikeno H, Ohmori K, Nishikawa T, Vollhardt J, Schehlmann V. Sodium ascorbyl phosphate shows in vitro and in vivo efficacy in the prevention and treatment of acne vulgaris. Int J Cosmet Sci 2005;27:171-6. [Google Scholar] |
| 105. | Woolery-Lloyd H, Baumann L, Ikeno H. Sodium L-ascorbyl-2-phosphate 5% lotion for the treatment of acne vulgaris: A randomized, double-blind, controlled trial. J Cosmet Dermatol 2010;9:22-7. [Google Scholar] |
| 106. | Apremilast in the Treatment of Moderate to Severe Acne. Identifier NCT01074502. Clinical Trials: A Service of the U.S National Institutes of Health. Available from: http://www.clinicaltrials.gov. [Last accessed on 2016 Jan 02]. [Google Scholar] |
| 107. | Owyang AM, Issafras H, Corbin J, Ahluwalia K, Larsen P, Pongo E, et al. XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1β-mediated diseases. MAbs 2011;3:49-60. [Google Scholar] |
| 108. | Reichert JM. Which are the antibodies to watch in 2013? MAbs 2013;5:1-4. [Google Scholar] |
| 109. | Lee WJ, Choi YH, Sohn MY, Lee SJ, Kim do W. Expression of inflammatory biomarkers from cultured sebocytes was influenced by treatment with Vitamin D. Indian J Dermatol 2013;58:327. [Google Scholar] |
| 110. | Kim BJ, Rho YK, Lee HI, Jeong MS, Li K, Seo SJ, et al. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin Dev Immunol 2009;2009:645898. [Google Scholar] |
| 111. | Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Törmä H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol 2005;124:1080-2. [Google Scholar] |
| 112. | Stotland M, Shalita AR, Kissling RF. Dapsone 5% gel: A review of its efficacy and safety in the treatment of acne vulgaris. Am J Clin Dermatol 2009;10:221-7. [Google Scholar] |
| 113. | Thorneycroft LH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis 2004;74:123-30. [Google Scholar] |
| 114. | Huber J, Walch K. Treating acne with oral contraceptives: Use of lower doses. Contraception 2006;73:23-9. [Google Scholar] |
| 115. | Shaw JC. Hormonal therapy in dermatology. Dermatol Clin 2001;19:169-78, ix. [Google Scholar] |
| 116. | Simonart T. Immunotherapy for acne vulgaris: Current status and future directions. Am J Clin Dermatol 2013;14:429-35. [Google Scholar] |
Fulltext Views
26,084
PDF downloads
7,007





