Translate this page into:
Upper face rejuvenation using botulinum toxin and hyaluronic acid fillers
Correspondence Address:
Soni Nanda
189 D, Garud Apartments Mayur Vihar, Phase 1, Delhi 110096
India
| How to cite this article: Nanda S, Bansal S. Upper face rejuvenation using botulinum toxin and hyaluronic acid fillers. Indian J Dermatol Venereol Leprol 2013;79:32-40 |
Abstract
The demand for facial rejuvenation is increasing, with each passing day, in all age groups. A number of procedures like chemical peels, microdermabrasion, laser and light therapies, and minimally invasive procedures like botulinum toxin injections (BTX A) and hyaluronic acid (HA) fillers are being extensively used by the dermatologist and plastic surgeons to meet this growing demand. A good knowledge of use of these techniques is becoming imperative for the dermatologist. In the present article, we discuss in detail the use of botulinum toxin injections and hyaluronic acid fillers for rejuvenation of upper face. Special emphasis has been placed on the complications associated with treatment of each area and on how to manage the same.Introduction
With the advent of minimally invasive cosmetic surgical techniques and the popularization of non-surgical interventions such as botulinum toxin (BTX A) injections and injectable fillers, it is not surprising that the demand for facial rejuvenation procedures is on the rise. This trend is not limited solely to the aging population. In fact, statistics have shown that the number of cosmetic operations performed in young adults aged 18 or younger have more than quadrupled from 13,300 to 81,000 during the past decade. [1]
The scope of the present article is to discuss the various indications, techniques, and complications associated with the use of BTX A and hyaluronic acid (HA) fillers, for rejuvenation of upper face. Before doing these procedures, the doctor should have a thorough knowledge of the facial anatomy and location of the underlying neuro-vascular structure. [2] A detailed description of the same is beyond the scope of the present article.
The face is divided according to the classical Da Vinci method into ′′horizontal thirds′′ from trichion to glabella (upper face), glabella to subnasale (mid face), and subnasale to menton (lower face) [Figure - 1]. The second method is advocated by some due to the variability of the hairline and is accomplished by considering only the middle and lower third of the face, measuring from nasion to the subnasale, and subnasale to menton, with the midface and lower face representing 43% and 57% of the facial height, respectively. [3]
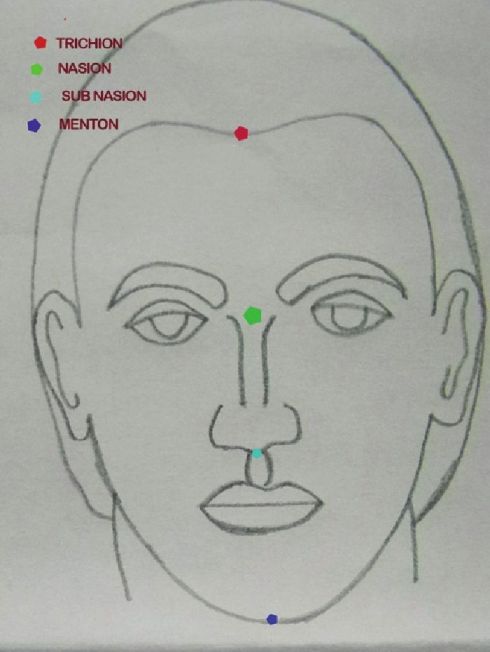 |
| Figure 1: Showing the division of face |
Analysis of the upper face consists of evaluating the quality of skin, the amount of photodamage, and skin aging according to the Glasgow scale in the forehead, brows, and eyelids. When performing facial analysis, we need to think of three components: The bony facial skeleton, the soft tissues, and the skin. Only 15% of human faces are perfectly symmetric. [4]
Botulinum toxin injections
BTX A injections have become one of the most effective and common aesthetic interventions carried out by physicians for upper face rejuvenation. The first report of BTX-A for cosmetic purposes was published in 1990 by Carruthers and Carruthers. [5] BTX A injection is a simple, safe, and effective treatment of the aging face, reducing wrinkles through the transitory and reversible paralysis of treated muscles. [6],[7] From being considered as a means to remove lines and wrinkles to a modality for giving a young and fresh look, BTX A treatments have seen a sea change in indications and techniques over the past few years.
Biology: BTX A is a neurotoxin, produced by a Gram-positive, spore-forming, obligate, anaerobic bacillus Clostridim botulinum. There are eight anti-genically distinct, but structurally and functionally similar cytoplasmic exotoxins identified as serotypes A, B, C1, C2, D, E, F, and G produced by C. botulinum, each having a molecular weight of about 150 kDa. [8] Neurotoxin A (BTX A) and B (Dysport) are being currently used for a number of cosmetic and therapeutic indications.
Storage: Vacuum-dried powdered BTX A should be frozen at -5°C until it is ready to be diluted with preservative free normal saline and administered. Reconstituted BTX A vials should be kept refrigerated at 2- 8°C at all times after reconstitution. [8],[9]
Reconstitution: Ideally, reconstitution should be done few hours prior to use with 0.9% sterile unpreserved, saline solution. Nonetheless, some studies showed that BTX A remains safe and effective for 2 to 6 weeks after reconstitution. [10] Preserved saline reduces the risk of bacterial contamination, hence increases the storage period. [9]
Dilution: In general, a higher concentration allows for more accurate placement and, therefore, greater duration of effect and fewer side effects. This is recommended for areas like lower face. Lower concentrations will encourage the spread of the toxin. Hence, this is recommended for areas like underarms. There is an area of denervation that is associated with each point of injection due to toxin spread (approximately 2.5 to 3.0 cm). The concentration of the toxin would be highest at the point of injection, and the concentration gradient decreases rapidly with distance from this point. [11] With higher dilutions, this area of diffusion increases and the concentration gradient becomes much less steep. Commonly used dilution for upper face injections is 2.5 ml preserved normal saline for 100 U vial and 1.3 ml for a 50 U vial.
Toxicity: The human LD50 (lethal dose) for a 70- kg person has been calculated to be approximately 40 U/ kg or about 2500-3000 U. The usual total dose of BTX A administered for cosmetic purposes during any treatment session is less than 100 U, which is about 3% of the LD50 for humans, making poisoning by accidental overdose highly unlikely and virtually impossible. [12],[13],[14]
Settings: BTX A injections are usually performed on an outpatient basis with the patient seated in a chair with a raised head support. Patient should be asked to stop NSAIDS, aspirin, or asprin-containing drugs and green tea one week before the procedure to decrease the risk of bruising. Physicians must evaluate the patient, analyzing the facial anatomy, observing patients at rest and contracting their muscles, and paying attention for any preexisting asymmetry. Photodocumentation with a standardized digital camera of the area to be treated, at rest and in animation is a must.
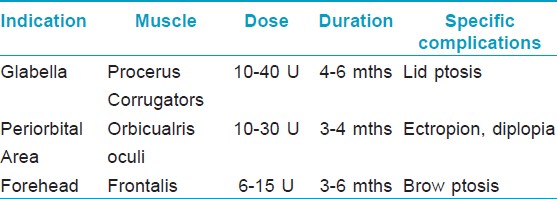
Makeup should be removed, and asepsis of the skin carried out using 70% isopropyl alcohol or iodine. Local anesthetic preparations or ice compresses can be used to decrease the pain. Suggested total doses of BTX A based on the consensus for BTX A dosage and other publications [15],[16] are 10- 40 U for glabella, 10-30 U for periorbital area, and 6-15 U for forehead. Patients must remain in the vertical position and avoid intense physical exercise and manipulating the injected area for at least 4 hours after injections. [17]
Specific Indications Glabella
Treatment of the glabella is the only FDA-approved cosmetic indication of BTX A. Treating this area removes the angry and tired look and gives a more pleasant and relaxed look to the face. Also, doing the BTX A injections at the right age will prevent the formation of static lines. Lewis and Bowler reported in their paper that BTX A cosmetic therapy correlates with a more positive mood. A psychological mechanism for this effect is reviewed, in which paralysis of the corrugator (frown) muscles leads to less facial feedback for negative emotions and hence correlates with reduced negative mood. [18] Horizontal frown lines are caused by procerus muscle while the contraction of corrugators supercilli muscles leads to vertical lines [Figure - 2].
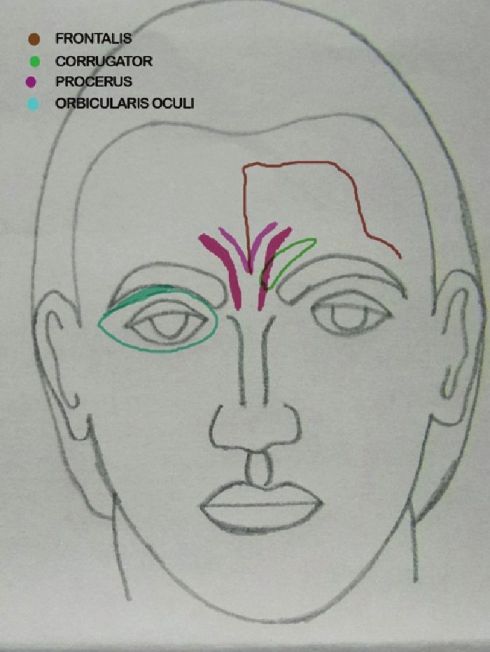 |
| Figure 2: Showing muscles of upper face |
Technique: Multiple injections (5 or 3 points) of relatively concentrated doses and low volumes in the procerus and the corrugators are recommended to treat this area. Intramuscular injection of BTX A at the midpoint of an imaginary "X" formed by lines joining the inner brows and the contralateral inner canthus into the procerus done. In patients with a long procerus muscle, the dose (6-12 U) can be divided in two points of this muscle. After palpating the medial aspect of the eyebrow as the patient squints and frowns, BTX A (6-8 U) is slowly injected into the belly of the corrugator muscle, keeping the needle tip pointed upward and away from the globe, at a distance of approximately 1 cm superior to the orbital rim [Figure - 3]. [8],[19]
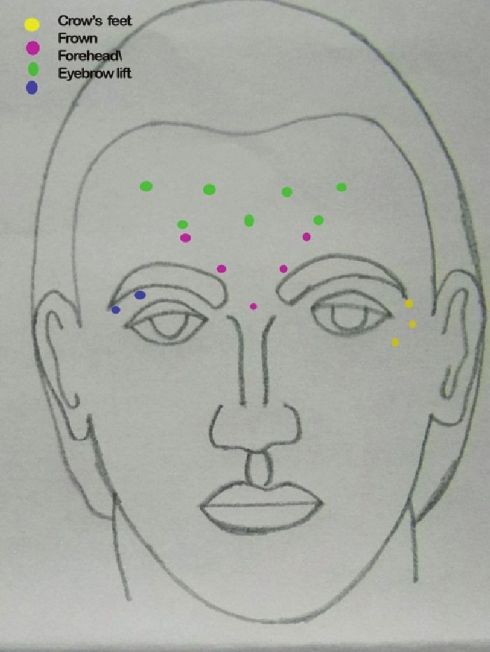 |
| Figure 3: Injection points for upper face BTX A |
The most difficult patients to treat seem to be those who possess thick sebaceous skin with deep wrinkles and furrows that are difficult to pull apart with the fingers. Patients who possess thinner, less sebaceous skin with finer wrinkles and shallower skin folds that can be spread apart and reduced with the fingers ("glabellar spread test") seem to have better results. [20]
Doses ranging from 10-40 U have been tried and found useful. [15],[16],[20] Dose depends on the muscle size. Males usually need higher doses than females. The duration of effect depends upon the muscle size, dose injected, and patient profile. It has been found that with 10 U, the result do not last long. [21] Overall the effect of BTX A is seen to last for 4-6 months and even more in some cases [Figure - 4] and [Figure - 5] [Table - 1].
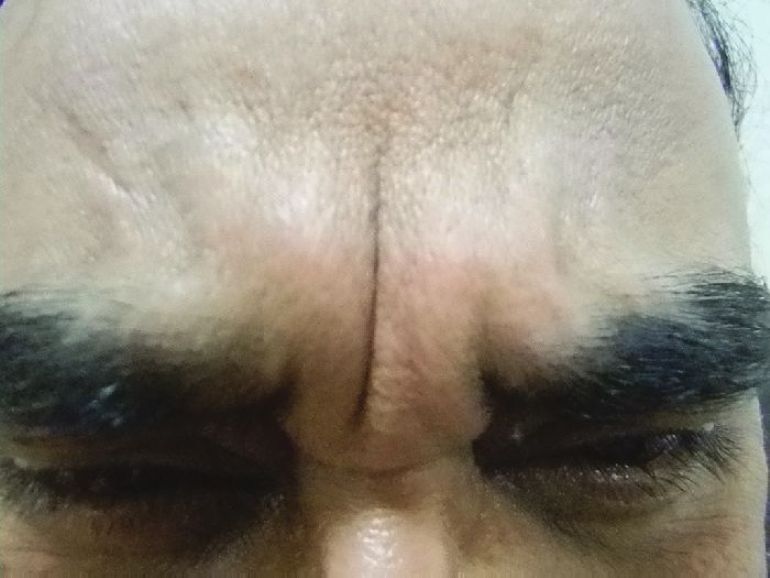 |
| Figure 4: Showing frown lines with a prominent Procerus |
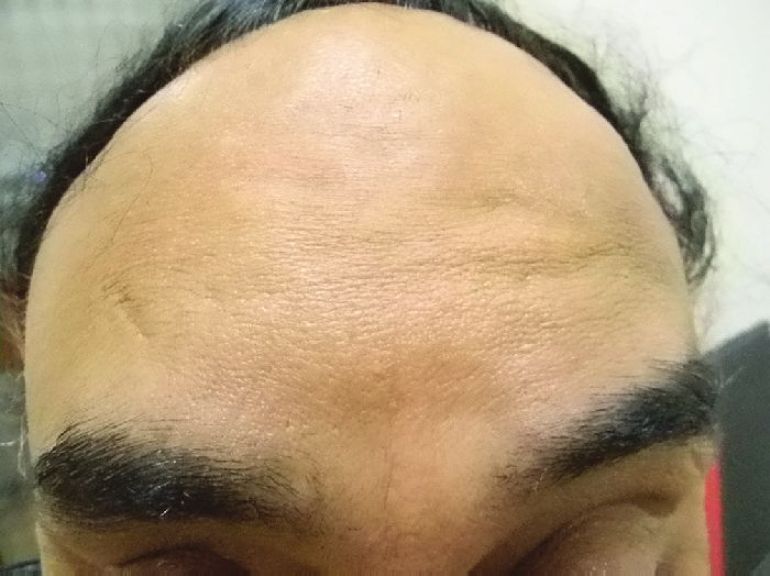 |
| Figure 5: 2 weeks after treatment with 40 U BTX A |

BTX A alone is a very good treatment option for patients having only dynamic lines. In those having both dynamic and static lines, the dynamic lines should first be treated with the required doses of BTX A. After 2 weeks, the patient is reassessed and fillers can be done for the static lines. Fillers have been found to stay for a longer duration when done post-BTX A injections as there is no or minimal muscle movement. Repeat BTX A injections at 4-6 months can also lead to an improvement in the static lines. BTX A is associated with fewer side effects as compared to fillers for this area.
Side Effects: Ptosis of the upper eyelid is the most significant and frequently occurring complication [Table - 1]. The 1 to 2 mm of ptosis is often initially only of subtle cosmetic significance, but as the day progresses, the eyelid droop becomes more exaggerated and more apparent. This is caused by the migration of injected BTX through the orbital septum, weakening the levator palpebrae superioris muscle. Ptosis can appear up to 7-10 days after a BTX injection and can last 2-4 weeks or longer. [8],[11],[22]
Ptosis occurs more frequently when large volumes of diluted BTX A are injected. An antidote for blepharoptosis is apraclonidine 0.5% eyedrops. The ocular instillation of this alpha-2-adrenergic agonist causes Muellers muscle to contract, temporarily raising the upper eyelid. One or two drops should be instilled into the affected eye. If ptosis persists after 20-30 min, additional one or two drops may be required for the desired effect. This procedure can be repeated three to four times a day. [23] Other common injection-related side effects in the glabellar area are headache, injection site pain, transitory edema and erythema or bruising, and nasopharyngitis. [24]
Periorbital area
Periorbital rhytides or crow′s feet wrinkles are radial lines mainly caused by the hyperactivity of the orbital portion of the orbiularis oculi muscle [Figure - 2]. [25]
Technique: Injections are performed in the subcutaneous plane, 1 cm lateral to the orbital rim at three sites (2-4 U/site) overlying the lateral fibers of the orbicularis oculi, avoiding injecting the zygomaticus [Figure - 3]. [26] Injection points and the doses can be much higher in males, especially those having a muscular face. Hexsel has demonstrated that when the orbicularis oculi is exceptionally hyperfunctional, causing deeply elongated and resistant crow′s feet, additional injection sites posteriorly placed in the temporal area can be beneficial. [27] 10-30 U of BTX A have been used and found to be effective in this area. The effect stays for about 3-4 months [Figure - 6] and [Figure - 7] [Table - 1].
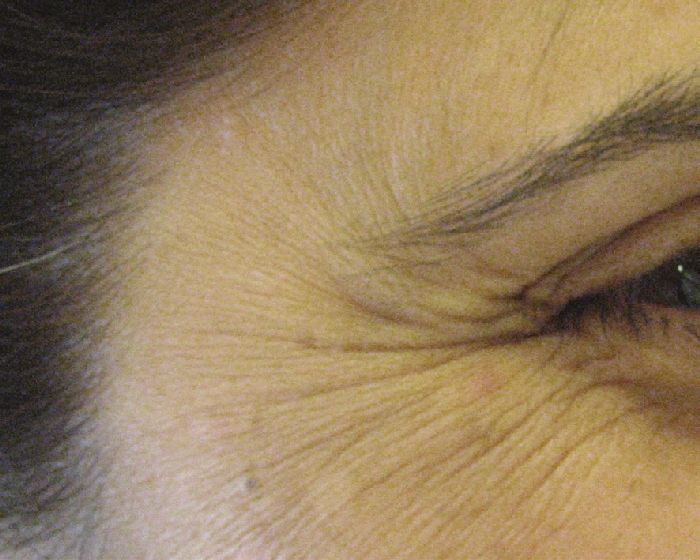 |
| Figure 6: Showing periorbital lines |
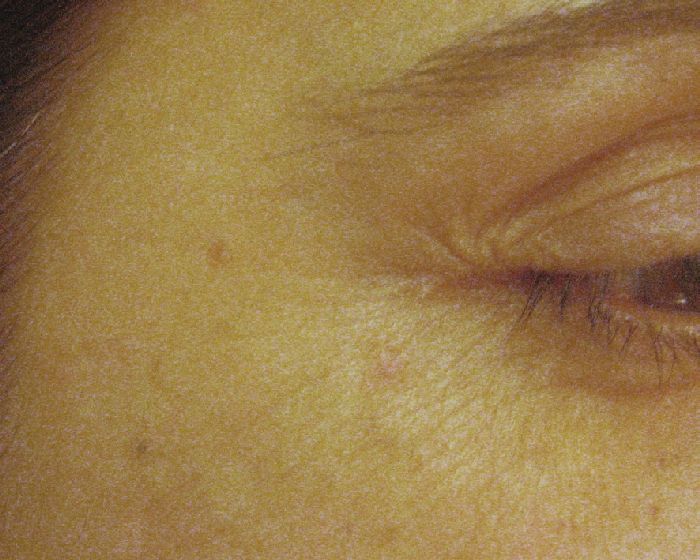 |
| Figure 7: After 2 weeks of 18 U BTXA injection |
Complications: Bruising, diplopia, ectropion or a drooping lateral lower eyelid, and asymmetric smile because of the injection in the zygomaticus major are the rarely encountered complications [Table - 1]. [28] BTX A should not be injected medial to an imaginary vertical line that passes through the lateral canthus, nor below the level of the superior margin of the zygomatic arch. Otherwise, the muscle fibers of some of the levators of the upper lip and corners of the mouth will be affected by the diffusion of the BTX, and result in upper lip ptosis.
Forehead
The frontalis muscle is an elevator muscle that lifts the forehead and eyebrows and is responsible for producing transverse forehead wrinkles [Figure - 2]. In majority of the individuals, the two bellies of frontalis are divided in the middle of the forehead by a central, tendinous aponeurosis, composed of little or no muscle fibers. In many males and some females, however, there are well-developed fibers in the center. These can be detected by light palpation over the area as the patient actively raises and lowers eyebrows. [29]
Technique: Any degree of pre-existing brow asymmetry should be noted before injection, and a warning should be given that this can be diminished or may be accentuated. [11] Injection points should be marked and then 1-2 U of BTX A should be placed subcutaneously or intramuscularly, at 1.5-2-cm intervals on either side of a deep crease in a "V′′ or horizontal configuration, starting approximately 1 cm above and at the medial side of the eyebrows and finishing at the hairline [Figure - 3]. When functional muscle fibers are present in the center of the forehead, injections at the midline should be given otherwise avoided. [29]
Three to four sites can be injected on either side of the midline for a total of six to eight sites for an entire forehead. [29] One finger breadth area above the orbital ridge lateral to the mid papillary line should be avoided [Figure - 3]. In order to avoid a high-arching brow, an additional 4-6 U of BTX A can be injected subcutaneously 1-1.5 cm above the supraorbital prominence at the mid pupillary line. A total of 6-15 U can be injected, and the result stays for 3-6 months [Figure - 8] and [Figure - 9] [Table - 1].
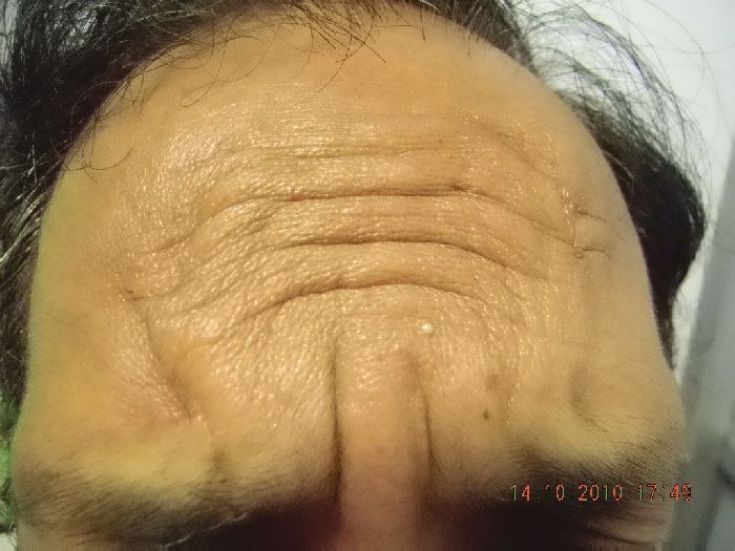 |
| Figure 8: Showing forehead and frown lines |
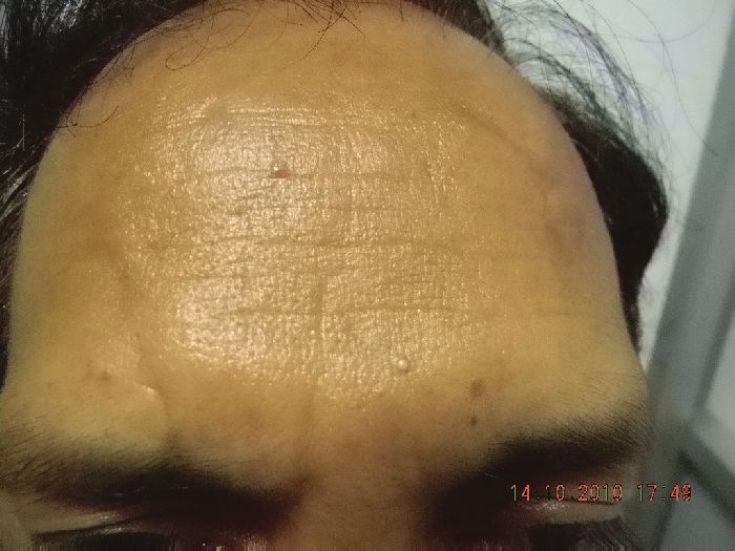 |
| Figure 9: Results seen 2 weeks after treatment with BTX A |
For some patients, the treatment of the upper part only is the best option because the treatment of the entire frontal region causes significant loss of facial expression (mask-like appearance).
An aesthetically unfavorable outcome is the brow that assumes a quizzical or ′′cockeyed′′ appearance [Figure - 10] [30] when bilateral a "Nicholson" brow is created. This occurs when the lateral fibers of the frontalis muscle have not been appropriately injected. For this reason, most individuals have moved their lateral forehead injections lateral to the iris and not the pupil. The treatment is to inject a small amount of BTX into the fibers of the lateral forehead that are pulling upward. Thus, in this situation, 3 U are injected medial to the temporal fusion line 2.5 cm above the orbital rim. [11]
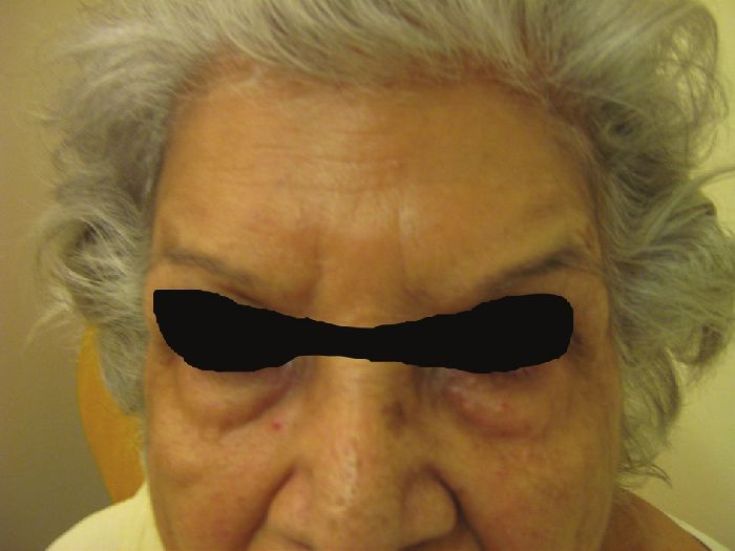 |
| Figure 10: Showing spockeyed appeareance after BTX A for forehead lines |
Brow ptosis is the site-specific complication [Table - 1]. This can be avoided by remaining 1.5-2 cm above the orbital ridge when injecting the frontalis lateral to the mid pupillary line with BTX A. This will enable the muscle fibers of the frontalis to remain functional in the area directly above the brow so as not to cause the eyebrows to droop and produce hooding over the upper eyelids. There is no antidote for brow ptosis, which can last as long as the causative injection is effective.
Injection of botulinum toxin into the lateral forehead, particularly the lower portion needs special expertise. Recently, Ghalamkarpour et al in a randomized clinical trial on 40 women showed that injection of BTX-A through the temporal hairline to correct supraciliary wrinkles seems to be safe and effective. [30]
Chemical brow lift
The ideal female brow position should begin medially at the level of an imaginary vertical line drawn through the alar base and medial canthus, arch superiorly 1 cm above the supraorbital rim and terminate laterally at a point on the line drawn from the alar base to the lateral canthus. In males, the arch of the brow should assume a more leveled appearance positioned at or slightly above the level of the supraorbital rim. Assessment of the resting brow position is important and can be achieved by having the patient close his or her eyes for 10 to 15 seconds prior to the examination. [31]
The eyebrow is elevated by the frontalis muscle and depressed by the medial brow depressors, corrugators supercilli muscle and procerus muscle, and the lateral brow depressors, lateral portion of orbicularis oculi [Figure - 2]. The paralysis of these muscles allows for unopposed brow abduction by the frontalis muscle.
Injections are given into the glabellar area and lateral orbital orbicularis muscle below the eyebrow. [32],[33] Further studies with more specific selection criteria are needed to better evaluate this effect. [34] To enhance the arching of the lateral eyebrow, especially in women, 2-4 U of BTX A can be injected into the lateral aspect of the orbital portion of the orbicularis oculi at a point at the lateral canthus, just above the supraorbital ridge and along the superior temporal line, which can be just above or below the hairs of the eyebrows, depending on the idiosyncratic anatomy of the patient being treated [Figure - 3]. This reduces the depressor action of the orbicularis oculi at the lateral aspect of the brow, allowing muscle fibers of the frontalis to elevate the lateral eyebrow [Figure - 11] and [Figure - 12]. [34]
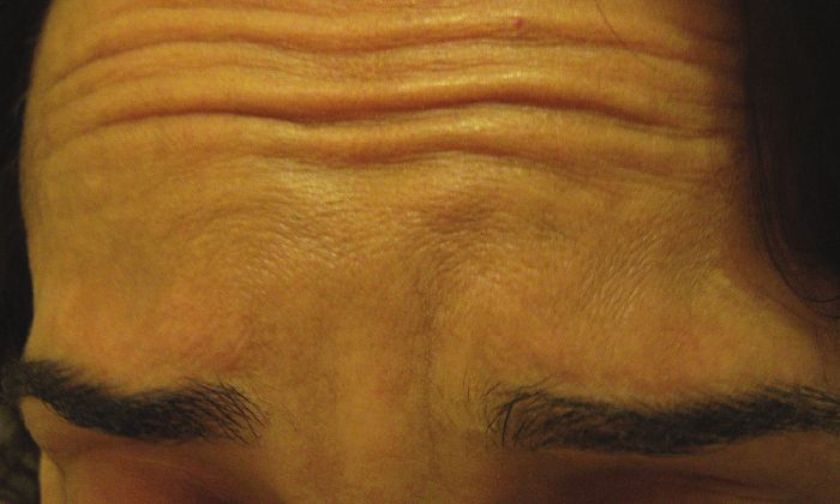 |
| Figure 11: Showing eyebrow lines and flat eyebrow shape |
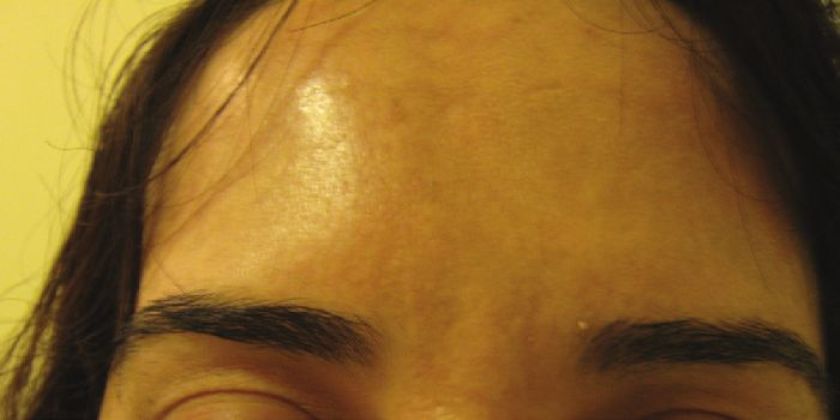 |
| Figure 12: After BTX A for forehead lines and chemical brow lift |
Contraindications
Contraindications for BTX-A include preexisting neuromuscular disorders (e.g. myasthenia gravis and amyotrophic lateral sclerosis); local infection at the expected injection site; known hypersensitivity to any component of the formulation; pregnancy; lactation; patients taking concomitant aminoglycosides or other substances interfering with neuromuscular transmission (e.g., curare-type non-depolarizing blockers, quinidine, magnesium sulfate, and succinylcholine) and patients with psychiatric disorders [Table - 2]. [8]
Complications
Complications associated with aesthetic use of BTX A are few and anecdotal. As most of the beneficial effects of BTX are temporary, fortunately, so too are the complications associated with this form of therapy. [35] Untoward sequelae that can occur at any site due to percutaneous injection of BTX include pain, edema, erythema, ecchymosis, headache, and short-term hyposthesia.
Discomfort can be decreased by use of topical anesthetics before injection and the use of smaller gauge needles. Ice applied immediately before and after injection will further reduce the pain as well as the edema and erythema associated with an intramuscular injection. Ecchymosis can be minimized by avoiding aspirin, aspirin-containing products, and products that inhibit platelet function (non-steroidal anti-inflammatory agents) for 7 to 10 days before injection. Limiting the number of injections and post-injection digital pressure without manipulation will assist in reducing the number and severity of bruising. Some physicians have advocated the use of homeopathic topical preparations containing vitamin K or arnica montana in hopes of further minimizing bruising. These agents have not really proven to be of benefit in BTX therapy. [11]
Although the onset of headaches has been initiated with BTX injections, they are for the most part alleviated with standard over-the-counter analgesics. It is, however, more common for patients to report that chronic tension headaches have been improved after injections of BTX. Furthermore, headache has occurred even when BTX is injected for hyperhidrosis. [36]
BTX A injections are not meant to replace upper, mid, or mandibular face and neck lifts; indeed, BTX A injections may optimize results from these surgical procedures. It has been suggested that to optimize the effect of the medical procedures, BTX A should be injected 3 weeks before surgery. In addition, BTX A injections may optimize and prolong the effect of the surface procedures as lasers, peels, and fillers. [37],[38],[39]
Hyaluronic acid fillers
Soft tissue augmentation of upper face, especially the glabellar line and the mid brow area, is becoming popular by the day. Of all the fillers available, hyaluronic acid (HA) fillers are the most commonly used fillers because of the low immunogenicity, high safety, and easy removal by hyaluronidase injections.
Of the many age-related changes in the face, volume loss in the glabella and forehead may combine with brow and eyelid ptosis and reduced lateral brow projection to give a fatigued and sad look. For facial lines resulting from the loss of volume associated with aging, injectable fillers, which efface and support the static rhytides, are the most suitable treatment. [40]
Volumizing the glabella and medial forehead can lift the medial brow, soften the horizontal forehead lines, and elevate the root of the nose, and in so doing, soften the horizontal procerus lines. HA is injected in the subgaleal glide plane between the brows at the mid procerus level between the supratrochlear vascular arcades, using a 1 ml syringe with a thin wall 28 G, 3/4 th needle. Slow and gentle anterograde injection technique with radial fanning is used. The procedure gives an immediate medial brow lift, the more open appearance of their palpebral apertures, the elevation of the nasal bridge, and the globally softer, more relaxed appearance of the periorbital region. Treatment typically lasts for at least 10 to 12 months (depending on the type of HA filler injected), although the duration of effect can be further extended if the subject has simultaneous treatment with BTX A. The mild lift that intradermal fillers produce augments the lift and aesthetic effect that can be achieved with BTX A alone. [41],[42],[43]
Non-cross-linked HA (IAL System) is used for giving hydration to the upper face. The filler is injected using a micropapular technique, and the effect lasts for about a month. Pain, bruising, and short longevity are the limiting factors of this procedure.
Erythema, swelling, and pain are the most frequent side effects seen after glabella injection. Although vascular compromise can occur in any location, glabella is the commonest area where skin necrosis can occur after injection. Blood supply of this area is from the small vessels branching from the supratrochlear and supraorbital arteries, and the collateral circulation is limited, hence glabella has been labeled as the danger area of the face due to chances of vascular compromise and subsequent necrosis. [44]
Venous occlusion can occur if excessive amounts of fillers are placed in a small area, leading to excessive venous congestion. This is associated with persistent, dull aching pain, and swelling with the development of a violaceous discoloration of the affected area. These findings can easily be misinterpreted and dismissed as early discomfort and bruising after treatment, but pain out of proportion (in terms of severity or persistence) to the treatment or for the individual patient should be further investigated. Nitroglycerin paste, warm compresses, and hyaluronidase injection have been found to be useful.
Direct arterial embolization of filler material generally causes an immediate skin blanching and pain, which may vary from severe to minimal. Injection should be stopped and aspiration should be attempted, which may relieve the blanching. Treatment of vascular occlusion should be swift and aggressive. [44],[45],[46] The area should be massaged (vigorously in the case of hyaluronic acid derivatives, to disburse the bulk of the filler) and warm compresses be applied to increase vasodilatation. Additionally, 2% nitroglycerine paste can be considered (based on the patient′s blood pressure status) to cause further vasodilatation. In this situation, injection of hyaluronidase may offer some benefit. [47]
One might also consider the use of hyperbaric oxygen in the case of dramatic vascular compromise and impending necrosis. Localized skin breakdown should be treated with topical (with or without systemic) antibiotics. Venous obstruction is typically associated with shallower skin breakdown, whereas arterial occlusion may lead to broad and deep skin loss. Conservative debridement should be performed when necessary. The patient should be followed closely and seen frequently to facilitate healing with minimal cosmetic deformity and to provide reassurance. Once healed, depressed scars and persistent erythema should be dealt with appropriately.
Although it may be impossible to prevent all episodes of vascular occlusion, several steps should be taken to minimize the risk of this occurrence, especially when treating the glabella. Small-gauge (30- or 32-G needles) should be used whenever possible and material injected with needle withdrawal. Complete awareness of injection plane may also reduce or eliminate this complication because product should be directed intra- rather than subdermally. The smallest possible volume capable of producing the desired effect should be injected in small, discrete aliquots. If this cannot be achieved with a reasonable volume, the patient should return in 7 to 14 days for additional correction. Proper pretreatment informed consent should discuss this uncommon complication. [44],[45],[46]
Conclusions
If a proper patient selection is done and the procedure is done keeping in mind the anatomical considerations and the standard guidelines for injections, BTX A and HA fillers can prove to be a very satisfying modality, both for doctor and for patient, for upper face rejuvenation.
| 1. |
McGrath MH, Schooler WG. Elective plastic surgical procedures in adolescence. Adolesc Med Clin 2004;15:487-502.
[Google Scholar]
|
| 2. |
Zoumalan RA, Larrabee WJ. Anatomic Considerations in the Aging Face. Facial Plastic Surg 2011;27:16-22.
[Google Scholar]
|
| 3. |
Karimi K, Adamson P. Patient Analysis and Selection in Aging Face Surgery. Facial Plast Surg 2011;27:5-15.
[Google Scholar]
|
| 4. |
Adamson PA, Zavod MB. Changing perceptions of beauty: A surgeon's perspective. Facial Plast Surg 2006;22:188-93.
[Google Scholar]
|
| 5. |
Carruthers A, Carruthers J. The treatment of glabellar furrows with botulinum A exotoxin. J Dermatol Surg Oncol 1990;16;83.
[Google Scholar]
|
| 6. |
Carruthers J, Carruthers A. Botulinum toxin in facial rejuvenation: An update. Dermatol Clin 2009;27:417-25.
[Google Scholar]
|
| 7. |
Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: Efficacy and safety. Dermatol Surg 2009;35:1893-901.
[Google Scholar]
|
| 8. |
Benedetto AV. The cosmetic uses of Botulinum toxin type A. Int J Dermatol 1999;38:641-55.
[Google Scholar]
|
| 9. |
Klein AW. Dilution and storage of Botulinum toxin.Dermatol Surg 1998;24:1179-80.
[Google Scholar]
|
| 10. |
Garcia A, Fulton JE. Cosmetic denervation of the muscles of facial expression with botulinum toxin.Dermatol Surg 1996;22:39-43.
[Google Scholar]
|
| 11. |
Klein AW. Complications, Adverse Reactions, and Insights with the use of botulinum toxin. Dermatol Surg 2003;29:549-56.
[Google Scholar]
|
| 12. |
Scott AB, Suzuki D. Systemic toxicity of botulinum toxin by intramuscular injection in the monkey. Mov Disord 1988;3:333-5.
[Google Scholar]
|
| 13. |
Meyer KF, Eddie B. Perspectives concerning botulism. Z Hyg Infektionskr 1951;133:255-63.
[Google Scholar]
|
| 14. |
Lamanna C, Hillowalla RA, Alling CC. Buccal exposure to botulinum toxin. J Infect Dis 1967;117:327-31.
[Google Scholar]
|
| 15. |
Carruthers JD, Glogau RG, Blitzer A. Advances in facial rejuvenation: Botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies - consensus recommendations. Plast Reconstr Surg 2008;121(5 Suppl.): 5S-30S; quiz 31S-6S.
[Google Scholar]
|
| 16. |
Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, Jaen P, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood unit) - part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol 2010;24:1278-84.
[Google Scholar]
|
| 17. |
Wollina U, Konrad H. Managing adverse events associated with botulinum toxin type-A: A focus on cosmetic procedures. Am J Clin Dermatol 2005;6:141-50.
[Google Scholar]
|
| 18. |
Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol 2009;8:24-6.
[Google Scholar]
|
| 19. |
Hankins CL, Strimling R, Rogers GS. Botulinum toxin for glabellar wrinkles. Dermatol Surg 1998;24:1181-83.
[Google Scholar]
|
| 20. |
Pribitkin EA, Greco TM, Goode RL, Keane WM. Patient selection in the treatment of glabellar wrinkles with botulinum toxin type A injection. Arch Otolaryngol Head Neck Surg 1997;123:321-6.
[Google Scholar]
|
| 21. |
Mangano A, Albertin A, La Colla L. A double-blind, randomized, placebo-controlled, two-dose comparative study of botulinum toxin type A for treating glabellar lines in Japanese subjects: What if sample size and statistical tests mattered.? Aesthetic Plast Surg 2009;33:788.
[Google Scholar]
|
| 22. |
Carruthers JD, Carruthers A. Botulinum A exotoxin in clinical ophthalmology. Can J Ophthalmol 1996;31:389-400.
[Google Scholar]
|
| 23. |
Matarasso SL. Complications of botulinum A exotoxin for hyperfunctional lines. Dermatol Surg 1998;24:1249-54.
[Google Scholar]
|
| 24. |
Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: Efficacy and safety. Dermatol Surg 2009;35:1893-901.
[Google Scholar]
|
| 25. |
Hexsel D, Hexsel CL. Botulinum toxins. In: Robinson JK, Hanke CW, Siegel DM, Fratila A, editors. Surgery of the skin-procedural dermatology, 2 nd ed. Edinburgh, UK: Elsevier; 2010. p. 433-46.
[Google Scholar]
|
| 26. |
Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow's feet: Double-blind ],[ placebo-controlled, dose-ranging study. Dermatol Surg 2009;35:1478-86.
],[ placebo-controlled, dose-ranging study. Dermatol Surg 2009;35:1478-86.'>[Google Scholar]
|
| 27. |
Kadunc BV. Periorbital wrinkles. In: D Hexsel, AT Almeida, editors. Cosmetic Use of Botulinum Toxin. Porto Alegre, Brazil: AGE Editora; 2002:149-50.
[Google Scholar]
|
| 28. |
Klein AW. Contraindications and complications with the use of botulinum toxin. Clin Dermatol 2004;22:66-75.
[Google Scholar]
|
| 29. |
Lehrer MS, Benedetto AV. Botulinum toxin - an update on its use in facial rejuvenation. J of Cosmet Dermatol 2005;4:285-97.
[Google Scholar]
|
| 30. |
Feily A, Fallahi H, Zandian D, Kalantar H. A succinct review of botulinum toxin in dermatology; update of cosmetic and noncosmetic use. J of Cosmet Dermatol 2011;10:58-67.
[Google Scholar]
|
| 31. |
Ho T, Brissett AE. Preoperative Assessment of the Aging Patient. Facial Plast Surg 2006;22:85-90.
[Google Scholar]
|
| 32. |
Huang W, Rogashefsky AS, Foster JA. Browlift with botulinum toxin. Dermatol Surg 2000;26:55-60.
[Google Scholar]
|
| 33. |
Huang SC, Carruthers A, Carruthers JD. Raising eyebrows with botulinum toxin. Dermatol Surg 2000;25:373-6.
[Google Scholar]
|
| 34. |
Frankel AS, Kamer FM. Chemical browlift. Arch Otolaryngol Head Neck Surg 1998;124:321-3.
[Google Scholar]
|
| 35. |
Klein A. Cosmetic therapy with botulinum toxin: Anecdotal memoirs. Dermatol Surg 1996;22:757-9.
[Google Scholar]
|
| 36. |
Alam M, Arndt KA, Dover JS. Severe, intractable headache after injection with botulinum. J Am Acad Dermatol 2002;46:62-5.
[Google Scholar]
|
| 37. |
Carruthers J, Carruthers A. The effect of full-face broadband light treatments alone and in combination with bilateral crow's feet botulinum toxin type A chemodenervation. Dermatol Surg 2004;30:355-66.
[Google Scholar]
|
| 38. |
Yamauchi PS, Lask G, Lowe NJ. Botulinum toxin type A gives adjunctive benefit to periorbital laser resurfacing. J Cosmet Laser Ther 2004;6:145-8.
[Google Scholar]
|
| 39. |
Tiereny EP, Hanke CW. Recent Advances in Combination Treatments for Photoaging: Review of the Literature. Dermatol Surg 2010;36:829-840.
[Google Scholar]
|
| 40. |
Carruthers J, Carruthers A. Volumising the glabella and forehead. Dermatol Surg 2010;36:1905-9.
[Google Scholar]
|
| 41. |
Carruthers J, Carruthers A, Maberley D. Deep resting glabellar rhytides respond to BTX-A and Hylan B. Dermatol Surg 2003;29:539-44.
[Google Scholar]
|
| 42. |
Carruthers A, Carruthers J. Botulinum toxin type A. J Am Acad Dermatol 2005;53:284-90.
[Google Scholar]
|
| 43. |
Carruthers JD, Glogau R, Blitzer A. Advances in facial rejuvenation: Botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies -consensus recommendations. Plast Reconstr Surg 2008;121:5-30.
[Google Scholar]
|
| 44. |
Glaich AS, Cohen JL, Goldberg LH. Injection Necrosis of the Glabella: Protocol for Prevention and Treatment After Use of Dermal Fillers Dermatol Surg 2006;32:276-81.
[Google Scholar]
|
| 45. |
Narins RS, Jewell M, Rubin M, Cohen J, Strobos J. Clinical conference: Management of rare events following dermal fillers-focal necrosis and angry red bumps. Dermatol Surg 2006;32:426-34.
[Google Scholar]
|
| 46. |
Sclafani AP, Fagien S. Treatment of Injectable Soft Tissue Filler Complications. Dermatol Surg 2009;35:1672-80.
[Google Scholar]
|
| 47. |
Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or Unwanted Hyaluronic Acid Misplacement. Dermatol Surg 2005;31:893-97.
[Google Scholar]
|
Fulltext Views
11,664
PDF downloads
3,095





