Translate this page into:
Upper genital tract infection due to Ureaplasma urealyticum: Etiological or syndromic management?
2 Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Somesh Gupta
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Dev T, Taneja N, Juyal D, Dhawan B, Gupta S. Upper genital tract infection due to Ureaplasma urealyticum: Etiological or syndromic management?. Indian J Dermatol Venereol Leprol 2017;83:489-491 |
Sir,
In light of the decreasing prevalence of traditional sexually transmitted pathogens, genital mycoplasma infections are gaining importance.[1] The pathogenic role of Ureaplasma spp. as a causative agent of upper and lower genital tract infections has been well established. If left undiagnosed, it can lead to sequelae such as pelvic inflammatory disease, infertility and ectopic pregnancy.[2],[3] However, its role is often underestimated due to the difficulty associated with isolating it. We report a case of cervical discharge due to infection of the upper genital tract with Ureaplasma urealyticum, which also resulted in secondary infertility.
A 32-year-old woman was referred to the sexually transmitted disease clinic, All India Institute of Medical Sciences, New Delhi, from the gynecology out-patient department, with a history of persistent vaginal discharge since 4–5 months. There was associated occasional dysuria along with pain and heaviness in the lower abdomen since 3–4 years. She had one 12-year-old living child and had suffered three miscarriages. Her menstrual cycles were regular. She was married for 15 years and denied having any pre- or extra-marital sexual contact. Her husband could not be contacted for eliciting history or for examination.
She was diagnosed to have reproductive tract tuberculosis 4 years back and had completed a 6 –month course of anti-tuberculosis treatment. Recent endometrial biopsy and aspirate did not show any granuloma or acid-fast bacilli. She was non-reactive for venereal disease research laboratories test and seronegative for human immunodeficiency virus, hepatitis B surface antigen and hepatitis C antibody.
Speculum examination revealed an odorless, mucoid, nonbloody, thick creamy discharge, from the cervical os. However, there was no erythema or inflammation of the cervical os or the vaginal walls, suggesting the source of discharge to be from the upper genital tract [Figure - 1]. Cervical motion tenderness was also absent. The perianal and perivulval areas were normal. There was no inguinal lymphadenopathy or suprapubic tenderness and her pelvic ultrasound was normal.
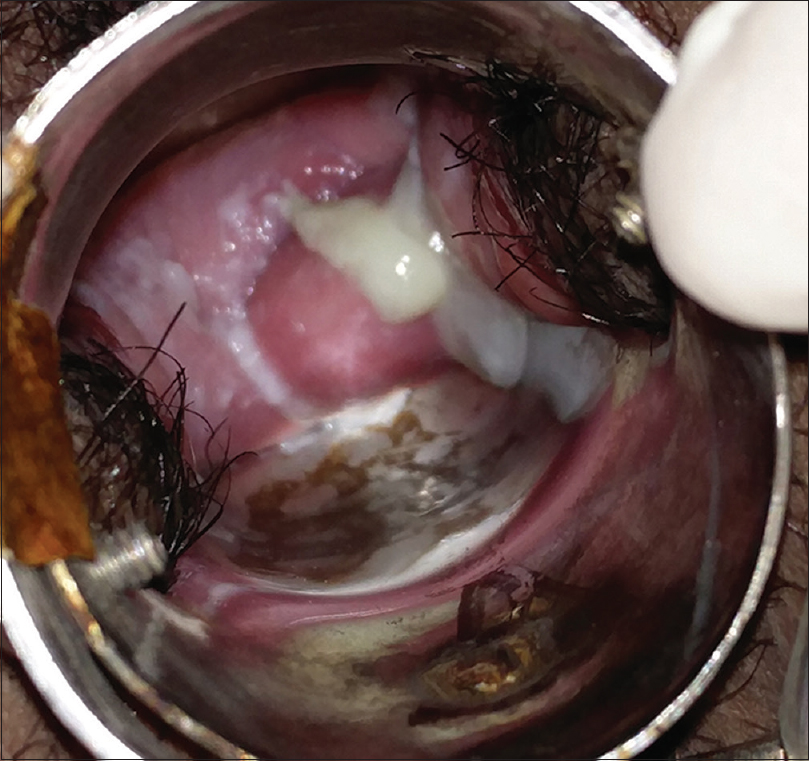 |
| Figure 1: Per-speculum view at initial presentation, showing moderate amount of creamy, mucoid discharge from the cervical os |
Gram-stained smear of the discharge showed 20–30 neutrophils per oil immersion field. No Gram-negative intracellular diplococci or any other pathogenic organisms were seen. The microscopic examination of cervical discharge by wet mount and 10% potassium hydroxide mount did not reveal any motile trophozoite, yeast cells or pseudohyphae. The patient was clinically diagnosed as having upper genital tract infection with cervical discharge. She was not given any syndromic treatment and was further investigated.
Three dacron swab specimens of the discharge were collected. Two swabs were inoculated into pleuro pneumonia-like organisms' broth containing urea and arginine for isolation of Ureaplasma spp. and Mycoplasma hominis, respectively. The semi-quantitative cultures were positive for Ureaplasma spp. at a concentration> 105 color changing units/ml within 48 h of incubation. The third swab was subjected to multiplex polymerase chain reaction for Ureaplasma spp. and M. hominis.[4] In addition, polymerase chain reaction for Mycoplasma genitalium and Chlamydia trachomatis was performed. The multiplex polymerase chain reaction was positive, only for Ureaplasma spp. which was further biotyped and was found to belong to biovar 2 (U. urealyticum).[5] Culture of the discharge was negative for Neisseria gonorrhoeae.
The patient was treated with doxycycline 100 mg twice daily for 14 days and her spouse was also prescribed azithromycin, 1 g as single dose to ensure compliance. She was also advised sexual abstinence till completion of treatment and was counseled regarding safe sexual practices afterward. On evaluation, after completion of treatment, there was remarkable improvement in her symptoms and repeat microscopy did not show any neutrophil.
Detection of Ureaplasma spp., although difficult, is possible by characteristic growth on appropriate culture medium, but biovar identification by molecular methods is important for the evaluation of pathogenicity.[6] Ureaplasma can also be isolated from healthy individuals and only certain subgroups of the species are pathogenic. Polymerase chain reaction is reported to offer a better diagnostic accuracy than culture; however, financial constraints limit its use in developing countries such as India.
The majority of human Ureaplasma isolates belong to biovar 1 (Ureaplasma parvum). Biovar 2 (U. urealyticum) is isolated less often and is found in the healthy human genitourinary tract. In a previous study, two authors have reported U. parvum as the predominant biovar in patients with genital tract infections.[1] In the absence of signs and symptoms of genital tract infections and the presence of other potential pathogens or commensals, a diagnosis of U. urealyticum-associated genital tract infections is difficult to make.[7] However, in our case, the presence of the characteristic discharge, microscopic findings, absence of other commonly found pathogens, culture of the organism and confirmation with polymerase chain reaction for U. urealyticum, along with the prompt response to specific treatment helped us to make a diagnosis of U. urealyticum associated upper genital tract infection.
Genital mycoplasmas including U. urealyticum are known to cause obstetric complications.[8] Recent studies have demonstrated a strong association between abnormal urogenital findings and the detection of U. urealyticum.[9] Schlicht et al. in their study found that 62% (40/65) of the total symptomatic males and females showed Ureaplasma exclusively on polymerase chain reaction and culture and were negative for all other organisms, hence implying causality.[9] Our patient also had a history of recurrent abortions and secondary infertility. As the patient's current investigations did not show any evidence of tuberculosis and cervical discharge tested positive for U. urealyticum, we speculated it to be the cause of infertility; however, we could not confirm it.
Treatment of Ureaplasma infection is imperative to prevent complications. Only some classes of antimicrobial agents (tetracyclines, macrolides and quinolones) are effective against Ureaplasma.[2] In a previous study from our center involving patients with infertility and genital discharge, 91%, 77% and 71% of Ureaplasma spp. isolates were susceptible to doxycycline, ofloxacin and azithromycin respectively, thus indicating that doxycycline should be the drug of choice when treating Ureaplasma infections.[2]
Currently, the focus is on syndromic management which is not a very sensitive and specific tool for diagnosing upper genital infection. Furthermore, because of ever increasing drug resistance, it is better to diagnose and treat the specific causative organism as far as possible. Although currently available only in higher centers, etiology-specific investigations such as culture and polymerase chain reaction should be made widely available. In a recent study from Western India, a total of 183 symptomatic pregnant females were treated syndromically and it was found that 78% were over-treated (false positive) and 19.1% were under-diagnosed (false negative); thus, indicating the low sensitivity and specificity of syndromic approach.[10]
Etiology-based treatment as against syndromic management of upper genital tract infections not only reduces irrational use of antimicrobials and subsequent antimicrobial resistance, but also helps clinicians in predicting the response to therapy. In case of persistent infection, the likelihood of resistance rather than misdiagnosis can be picked up more confidently.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gupta V, Dhawan B, Khanna N, Agarwal N, Bhattacharya SN, Sreenivas V, et al. Detection and biovar discrimination of Ureaplasma urealyticum in Indian patients with genital tract infections. Diagn Microbiol Infect Dis 2008;60:95-7.
[Google Scholar]
|
| 2. |
Dhawan B, Malhotra N, Sreenivas V, Rawre J, Khanna N, Chaudhry R, et al. Ureaplasma serovars & their antimicrobial susceptibility in patients of infertility and genital tract infections. Indian J Med Res 2012;136:991-6.
[Google Scholar]
|
| 3. |
Skiljevic D, Mirkov D, Vukicevic J. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in genital samples collected over 6 years at a Serbian university hospital. Indian J Dermatol Venereol Leprol 2016;82:37-41.
[Google Scholar]
|
| 4. |
Stellrecht KA, Woron AM, Mishrik NG, Venezia RA. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J Clin Microbiol 2004;42:1528-33.
[Google Scholar]
|
| 5. |
De Francesco MA, Negrini R, Pinsi G, Peroni L, Manca N. Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Clin Microbiol Infect Dis 2009;28:641-6.
[Google Scholar]
|
| 6. |
Saigal K, Dhawan B, Rawre J, Khanna N, Chaudhry R. Genital Mycoplasma and Chlamydia trachomatis infections in patients with genital tract infections attending a tertiary care hospital of North India. Indian J Pathol Microbiol 2016;59:194-6.
[Google Scholar]
|
| 7. |
Jansen JS. Genital mycoplasmas. In: Gupta S, Kumar B, editors. Sexually Transmitted Infections. New Delhi: Elsevier; 2012. p. 569-77.
[Google Scholar]
|
| 8. |
Gupta A, Gupta A, Gupta S, Mittal A, Chandra P, Gill AK. Correlation of mycoplasma with unexplained infertility. Arch Gynecol Obstet 2009;280:981-5.
[Google Scholar]
|
| 9. |
Schlicht MJ, Lovrich SD, Sartin JS, Karpinsky P, Callister SM, Agger WA. High prevalence of genital mycoplasmas among sexually active young adults with urethritis or cervicitis symptoms in La Crosse, Wisconsin. J Clin Microbiol 2004;42:4636-40.
[Google Scholar]
|
| 10. |
Shah M, Deshmukh S, Patel SV, Mehta K, Marfatia Y. Validation of vaginal discharge syndrome among pregnant women attending obstetrics clinic, in the tertiary hospital of Western India. Indian J Sex Transm Dis 2014;35:118-23.
[Google Scholar]
|
Fulltext Views
9,706
PDF downloads
2,330





