Translate this page into:
Use of fine needle aspirate from peripheral nerves of pure-neural leprosy for cytology and PCR to confirm the diagnosis: A pilot study
2 Department of Dermatology, Institute of Post Graduate Medical Education and Research, Kolkata, West Bengal, India
3 Department of Neuropathology, Bangur Institute of Neurology, Kolkata, West Bengal, India
4 Department of Dermatology, Sikkim Manipal University, Sikkim, India
Correspondence Address:
Abhishek De
Department of Dermatology, SSKM Hospital, Kolkata, West Bengal
India
| How to cite this article: Reja AH, De A, Biswas S, Chattopadhyay A, Chatterjee G, Bhattacharya B, Sarda A, Aggarwal I. Use of fine needle aspirate from peripheral nerves of pure-neural leprosy for cytology and PCR to confirm the diagnosis: A pilot study. Indian J Dermatol Venereol Leprol 2013;79:789-794 |
Abstract
Background: The diagnosis of pure neural leprosy (PNL) remained subjective because of over-dependence of clinical expertise and a lack of simple yet reliable diagnostic tool. The criteria for diagnosis, proposed by Jardim et al., are not routinely done by clinicians in developing country as it involves invasive nerve biopsy and sophisticated anti-PGL-1 detection. We conducted a study using fine needle aspiration cytology (FNAC) coupled with Ziehl Neelsen staining (ZN staining) and Multiplex- Polymerase Chain Reaction (PCR) specific for M. leprae for an objective diagnosis of pure neural leprosy (PNL), which may be simpler and yet reliable. Aim: The aim of the study is to couple FNAC with ZN staining and multiplex PCR to diagnose pure neural leprosy patients rapidly, in simpler and yet reliable way. Methods: Thirteen patients of PNL as diagnosed by two independent consultants were included as case, and 5 patients other than PNL were taken as control in the study. Fine needle aspiration was done on the affected nerve, and aspirates were evaluated for cytology, ZN staining and multiplex- PCR. Results: Out of the 13 cases where fine needle aspiration was done, M. leprae could be elicited in the nerve tissue aspirates in 5 cases (38.4%) with the help of conventional acid-fast staining and 11 cases (84.6%) with the help of multiplex PCR. On cytological examination of the aspirates, only 3 (23%) cases showed specific epithelioid cells, whereas 8 (61.5%) cases showed non-specific inflammation, and 2 (15.3%) cases had no inflammatory cells. Conclusion: Our study demonstrates that in the field of laboratory diagnosis of PNL cases, FNAC in combination with ZN staining for acid-fast bacilli (AFB) and Multiplex-PCR can provide a rapid and definitive diagnosis for the majority of PNL cases. FNAC is a less-invasive, outdoor-based and simpler technique than invasive nerve biopsy procedure. Thus, this study may enlighten the future path for easy and reliable diagnosis of PNL.Introduction
Leprosy patients lacking skin lesions, but showing involvement of one or more nerves, are afflicted with pure neural leprosy (PNL). On an average, PNL accounts for 5-17.7% of all leprosy cases and is particularly difficult to diagnose as acid-fast bacilli are usually not found in the skin smear or the histological section of nerves. [1] In the absence of a simple non-invasive and sensitive test, the diagnosis of PNL remains clinical and subjective.
Although no such specific diagnosis tool is being put forward neither by any international body nor by any national body with a high specificity and sensitivity, so far some are with high sensitivity and some with high specificity. Hence, combining various tests may improve the precision of diagnostic procedure of PNL. The gold standard for PNL diagnosis is so far considered as the histopathological examination of a peripheral nerve biopsy. Even so, the detection of bacteria is difficult and histopathological finding may be non-specific. Furthermore, nerve biopsy is an invasive procedure and that is only possible in specialized centers. [2] Moreover, as leprosy commonly involves important motor or mixed nerves like ulnar, median, common peroneal nerve, which are unsafe for biopsy even when it is done by an expert. Again nerve biopsy is often unproductive even if it is performed from the relatively safer sural or radial cutaneous nerve. [3] To address this issue, recently fine needle aspiration cytology (FNAC) of the affected nerves has emerged as an alternative method of diagnosis. [4],[5]
Previously, in an approach to diagnose PNL, Jardim et al. proposed a diagnostic criteria, which involved nerve biopsy, cytology, Polymerase chain reaction (PCR), and estimation of anti-phenolic glycolipid - 1 (anti PGL - 1). [6] However, these criteria remained widely unpopular in this part of the world as it involves highly skilful invasive nerve biopsy and sophisticated anti-PGL 1 detection.
With this background, this study is aimed to find out the effectiveness of combining FNAC with cytology, ZN staining and multiplex PCR technique in early, rapid, simpler and possible accurate diagnosis of PNL. As these investigations are safe, ethical, sensitive and do not involve delaying nerve biopsy, we presumed that this study may enlighten the future path for rapid and early and objective diagnosis of PNL.
Methods
Patient selection criteria
Thirteen clinically suspected cases of PNL were included in the study after obtaining a written informed consent form. The clinical diagnosis was done by two independent experienced dermatology consultants. For clinical diagnosis, definition of PNL was taken as thickening and/or tenderness of a peripheral nerve commonly involved by leprosy with sensory and/or motor functional impairment along the distribution of same nerve. These patients did not have any skin changes suggestive of leprosy.
Patients who were diagnosed by both the clinicians independently as cases of PNL were included in a group designated as "Possible PNL." Others who were diagnosed as PNL by one observer and non-PNL by the other observer were included in a group designated as "Doubtful PNL." The presence of "Doubtful group" even in a regional highest referral center like ours, shows that there is an element of subjectivity even amongst the most experienced and skilful dermatologists. Patients who had no clinical sign of Hansen neuropathy, but had easily identifiable ulnar nerve, were included as the "Control group," provided they signed the informed consent form.
Nerve conduction study
All the patients were subjected to nerve conduction study (NCS) as per the standard protocols of our hospital in the department of neurosciences.
Fine needle aspiration
Fine needle aspiration (FNA) was done as described by Theuvenet et al., [7],[8] and aspirates were subjected to cytological examination with Giemsa and ZN staining.
Extraction of DNA from the FNA samples and multiplex PCR
Genomic DNA was extracted from a portion of the FNA sample aspirates, collected aseptically with the standard precautions from ulnar nerve of study subjects by standard Phenol Chloroform method after proteinase-K digestion as described by Banerjee et al.[9]
Multiplex PCR
A multiplex PCR for the rapid diagnosis of M. leprae was performed as per Banerjee et al., [10] based on the following oligonucleotide primers sets:
- The repetitive sequence of the M. leprae DNA reported by Han et al., [11] is very specific to M. Leprae and not present in 20 other mycobacterial species other than M. Leprae. (Primer LR1 and LR2) [12] as shown in [Table - 1].
- A region flanking entire 21TTC repeat sequences, specific for multibacillary leprosy (MB) designed by Shin et al.[12] The specificity and sensitivity of the primers: LR1 and LR2 and TTC-A and TTC-B had been already established in our earlier studies. [10]
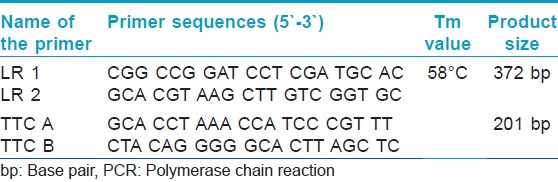
Briefly, 100 ng genomic DNA was amplified with Ampli Taq Gold, (Applied Biosystems, Inc. [ABI], Foster City, CA) in PCR reaction mixtures, containing 1x PCR buffer (Applied Biosystems), 2 mM MgCl2, 0.25 mM each dNTP, 20 picomoles primers LR1 and LR2 and TTC-A and TTC-B. The primer sequences, primer annealing temperature (Ta°C), and PCR product sizes are given in [Table - 1]. The PCR reactions were performed in the following conditions: 95°C for 4 minutes, followed by 35 cycles of 95°C for 1 min, Ta° for the internal control as given in [Table - 1] for 1 min, 72°C for 1 min, and finally elongated at 72°C for 10 minutes. The amplified products were separated by electrophoresis on 2% agarose gel stained with 0.5 mg/mL ethidium bromide and visualized and photographed under a UV transilluminator.
Results
The study included a total of 13 cases as "Study group" and 5 cases as "Control group." Out of the 13 cases, 9 patients were suggested as Hansen neuropathy by at least one of our two observers and were included in "Probable PNL" group, and 4 were included in "Doubtful PNL" group as per inclusion criteria of our study. Out of the 5 patients in "Control group" 1 patient had diabetic neuropathy and the other 4 patients were daily laborer, among which 1 patient had traumatic nerve injury with easily noticable and identifiable right ulnar nerve.
Out of the 13 cases as in [Table - 2], 12 patients were male and 1 patient was female within an age group of 15-45 years. Ten patients had both sensory and motor nerve involvement with 3 of them having grade 1 and 7 having grade 2 deformity as given in [Table - 2], whereas 3 patients had only sensory nerve involvement. None of the patients showed any sign of reactions.

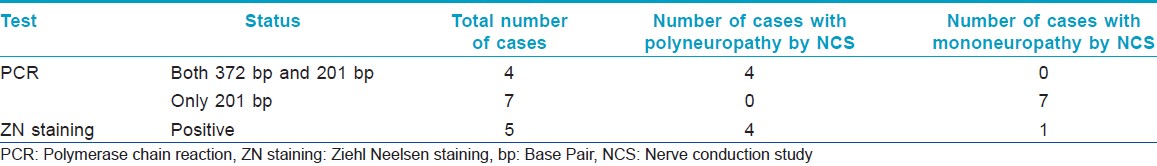
On nerve conduction study (NCS) as shown in [Table - 2], out of the 4 "Doubtful PNL," 2 patients had normal NCS and other 2 patients had deranged NCS. However, all 9 "Probable PNL" cases showed some form of defective nerve conduction. Eleven patients who showed abnormal NCS, all showed features of axonopathy, whereas 1 patient also had features of associated demyelinating neuropathy. On combining clinical and neurological findings, we had 11 patients with features of neuropathy, of which 7 had mononeuropathy and 4 had polyneuropathy.
FNAC of all the 9 "Probable PNL" patients showed presence of inflammatory cells in nerve aspirates, whereas out of the 4 "Doubtful PNL," only 2 cases showed inflammatory infiltrate and the rest had no signs of inflammation. However, out of the 11 patients, who had inflammatory aspirates, only 3 had presence of epithelioid cells, whereas all others had non-specific inflammation like neutrophils and lymphocytes. None of our patients had presence of foamy macrophages in their nerve aspirates as given in [Table - 2].
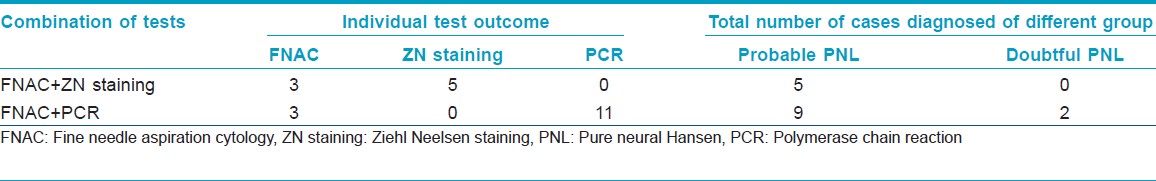
In case of ZN staining of the nerve aspirate as in [Figure - 1], out of the 13 cases, we found 5 cases positive for the presence of M. leprae, while the rest 8 cases were found negative for AFB as shown in [Table - 2]. All of the 5 patients positive for AFB were within "Probable PNL" group, whereas none of the "Doubtful PNL" showed presence of M. leprae as in [Table - 3].
 |
| Figure 1: Multiplex PCR showing Lane 1: PhiX174 DNA Marker. Lane 2, 4, 7, 8: Shows positive bands for both 372 bp and 201 bp. Lane 3, 5, 6, 9, 10: Shows positive band for 201 bp only. Lane 11: Positive control (Thai 53 strain) Lane 12: Negative control |
Multiplex-PCR when conducted to the aspirations of "Probable PNL" cases showed positive results in all 9 samples. However, out of the 4 "Doubtful PNL," only 2 cases were found positive and the rest 2 were found negative for the presence of M. leprae as shown in [Table - 2]. Out of the 9 patients, who showed positive PCR, 4 cases showed positive bands for both 372 bp and 210 bp, whereas the rest 5 cases showed positive band only for 201 bp as shown in [Figure - 2]. The 2 "Doubtful PNL" found positive for multiplex-PCR showed positive band only for 201 bp.
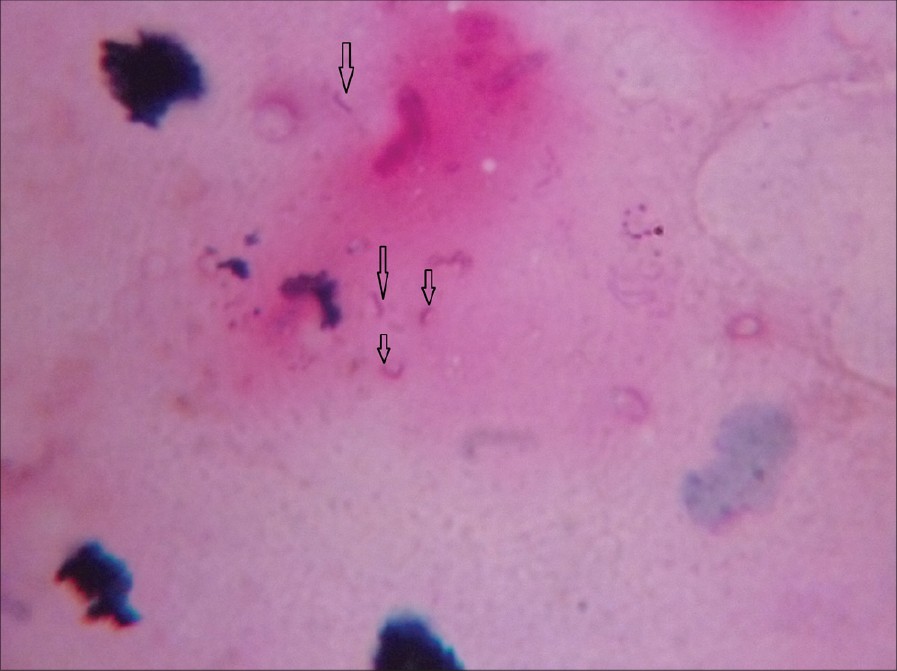 |
| Figure 2: FNAC aspirates subjected to ZN staining (Carbol Fuchsin stained counter stained by Methylene Blue viewed under X100 immersion oil). Arrows showing AFB mostly fragmented |
In case of "Control group," all 5 patients with easily identifiable nerves were found negative for PCR, ZN staining, and FNAC. Among them, 1 patient had diabetic neuropathy and the rest 4 were daily laborer with prominent ulnar nerves; among these 4 patients, 1 had traumatic nerve injury. Negative results in control group gave excellent reference for our Multiplex PCR study.
Discussion
Incidence of pure neural leprosy in India has been reported to range from 5.5% to 17.7% of all leprosy cases. [1] Though this constitutes a considerably large fraction of total patients of leprosy, diagnosis of PNL has been purely subjective in the absence of a rapid and yet simple less-invasive laboratory investigation. We, in this study, tried to find out a simple yet effective objective diagnostic tool of PNL by combining PCR AFB staining with FNAC.
Electrophysiological studies such as NCS in our patients showed that 11 out of 13 patients in the study group (84.6%) had axonal neuropathy and only 1 (7%) had features of demyelinating neuropathy as given in [Table - 2]. This result correlates well with Jardim et al. [6] whose findings were 91% and 8%, respectively. This simple technique not only confirms the peripheral neuropathy but also could be valuable instrument in choosing appropriate nerve for FNA. However, as axonal neuropathy of peripheral nerves may occur in a number of conditions, it hardly can be valued as an objective diagnosis of leprosy neuropathy.
The cytological study of the FNA can also provide some supportive evidence of leprosy neuropathy. [5] In our study, we preferred FNAC over biopsy as it was minimally invasive procedure, required very little expertise, can be performed in out-patient department and also minimize the risk of neural damage.
Singh et al. from India documented the cytomorphologic features of leprous neuritis from nerve aspirates of 28 patients. They concluded that the entire spectrum of leprosy is seen in nerve aspirates. [13]
However, Jardim et al. observed, the defining histological criteria for leprosy neuropathy should include the presence of AFB and epithelioid granuloma, and the presence of a non-specific inflammatory infiltrate (mononuclear cells with no differentiation as to Virchow or epithelioid cells) cannot be taken as a specific diagnostic finding of PNL. They documented though presence of non-specific inflammation was observed in about 71% of the patients, only 40% cases had definitive diagnostic features like AFB or epithelioid granuloma or both. [6]
In our study, out of the 13 suspected cases when subjected to FNAC, we found specific epithelioid cells in only 3 (23%) cases, non-specific inflammation in 8 (61.5%) cases, and 2 (15.3%) cases had no inflammatory cells in the aspirates. Similarly, when FNAC aspirates were subjected for ZN staining as shown in [Figure - 1], only 5 (38.4%) cases had AFB positive as shown in [Table - 2], whereas all others were AFB-negative. Based on this data, we could confirm 5 (38.4%) cases as PNL when AFB and cytological data were collaborated together as in [Table - 3]. All of these 5 (38.4%) cases were in "Probable PNL" group as shown in [Table - 3]. Moreover, out of the 5 AFB-positive cases, 4 cases were having polyneuropathy while the remaining 1 was having mononeuropathy as shown in [Table - 4].
Our findings closely matched that of Jardim et al.[6] and a relative low yield in both our studies points out at the limitation of using either cytological findings or AFB staining as sole diagnostic criteria of PNL.
In the recent years, PCR technique has been successful in demonstrating the presence of M. leprae DNA in leprosy patient′s samples. [9] In a study conducted from India, diagnostic sensitivity of PCR was found to be 88% when DNA was extracted from the biopsy samples of skin patches. [14]
Jardim et al., [6] in their study, used PCR to diagnose cases, in which the clinical and the histopathological data could not confirm PNL. Their findings were consistent with the results of Chemoulli et.al. in which PCR in nerve specimens increased the frequency of M. leprae detection. [15] However, till date, the PCR was mostly done from nerve biopsy specimens. Nerve biopsy is an invasive and highly specialized procedure and ethically may not be permitted as routine investigation, especially when done in the important motor or mixed nerves. [16]
We tried to combine relatively less-invasive and simpler FNAC technique and PCR from FNAC aspirate to overcome the above-mentioned challenges. To the best of our knowledge, our study is the first effort to combine these two procedures to suggest a simpler yet effective objective tool for diagnosing PNL.
In our study, PCR was positive in nerve aspirates of 11 out of 13 (84.6%) suspected cases as shown in [Figure - 2]. The only 2 cases which had negative PCR were in the doubtful group. They also had negative NCS, cytology and ZN staining as shown in [Table - 2].
We also found, 4 out of the 9 PCR-positive cases as shown in [Table - 2] had positive bands for both 372 bp and 201 bp, suggestive of high load of bacteria in patient as shown in [Figure - 2]; also when compared with NCS, the outcome showed to have a correlation with polyneuropathy as shown in [Table - 4], while the other 7 cases including the 2 "doubtful cases" showed positive band for only 201 bp, suggestive of low bacterial count in patients, and when compared with NCS, the outcome showed to have a correlation with mononeuropathy as shown in [Table - 4]. Hence, PCR from FNAC may also be suggestive of bacterial load and status of neuropathy in the patient body.
Moreover, a widely held belief to confirm diagnosis of doubtful diseases like PNL, one should diagnose the disease first with a sensitive test followed by the occurrence of positive result with a specific test. Hence, it is clear that by combining FNAC with ZN staining for the presence of AFB (Sensitivity 60%) and PCR (Specificity 100%), we could confirm all 9 cases who were in the "Probable PNL" group and 2 out of 4 cases who were in the "Doubtful PNL" group as given in [Table - 3]. Hence, out of 13 cases, 11 (84.6%) cases were successfully diagnosed for PNL as shown in [Table - 3]. Out of the 2 negative cases among the "Doubtful PNL" group, 1 was female, turned out to have osteomyelitis, mal-union, and entrapment neuropathy;′ she was sent to orthopedics and was treated surgically while the other was an out-patient from the outpatient department of one of the observer who initially diagnose as Hansen, decided to carry with his clinical judgment despite of his negative results and prescribed him with MDT PB.
Conclusion
Based on the above discussion, we may conclude that in the field of laboratory diagnosis, FNAC in combination with Ziehl-Neelsen staining for AFB and Multiplex-PCR can provide a rapid, reliable, efficient and definitive diagnosis for the majority of PNL cases. Thus, this study may enlighten the future path for easy and reliable diagnosis of PNL and probably reinforce the leprosy elimination process.
| 1. |
Giridhar BK. Neuritic leprosy. Indian J Lepr 1996;68:35-42.
[Google Scholar]
|
| 2. |
Jardim MR, Antunes SL, Simons B, Wildenbeest JG, Nery JA, Illarramendi, et al. Role PGL - I antibody detection in the diagnosis of pure neural leprosy. Lepr Rev 2005;76:232-40.
[Google Scholar]
|
| 3. |
Smith EW. Diagnosis of pure neuritic leprosy. Neurol J Southeast Asia 2002;7:61-3.
[Google Scholar]
|
| 4. |
Prasad PV, George RV, Kaviarasan PK, Viswanathan R, Tippoo R, Anandi C. Fine needle aspiration cytology in leprosy. Indian J Dermatol Venerol Leprol 2008;74:352-6.
[Google Scholar]
|
| 5. |
Siddaraju N, Sistla SC, Singh N, Muniraj F, Chahwala Q, Basu D, et al. Pure neritic leprosy with nerve abscess presenting as a cystic, soft tissue mass: Report of a case diagnosed by fine needle aspiration cytology. Diagn Cytopathol 2009;37:355-8.
[Google Scholar]
|
| 6. |
Jardim MR, Antunes SL, Santos AR, Nascimento OJ, Nery JA, Sales AM, et al. Criteria for diagnosis of pure neural leprosy. J Neurol 2003;250:806-9.
[Google Scholar]
|
| 7. |
Theuvenet WJ, Miyazaki N, Roche P, Shrestha I. Cytological needle aspiration for the diagnosis of pure neural leprosy. Indian J Lepr 1996;68:109-12.
[Google Scholar]
|
| 8. |
Theuvenet WJ, Miyazaki N, Roche P, Shrestha I. Cytological needle aspiration of the nerve for the diagnosis of pure neural leprosy. Int J Lepr Other Mycobact Dis 1993;61:597-9.
[Google Scholar]
|
| 9. |
Banerjee S, Sarkar K, Gupta S, Mahapatra PS, Gupta S, Guha S, et al. Multiplex PCR technique could be an alternative approach for early detection of leprosy among close contacts: A pilot study from India. BMC Infect Dis 2010;10:252-5.
[Google Scholar]
|
| 10. |
Banerjee S, Ray D, Bandopadhyay D, Gupta S, Gupta S, Ghosal C, et al. Development and application of a new efficient and sensitive Multiplex Polymerase Chain Reaction (PCR) in a diagnosis of leprosy. J Indian Med Assoc 2008;106:436-40.
[Google Scholar]
|
| 11. |
Han YK, Nae CS, Kyeong LM. Evaluation of polymerase chain reaction amplification of Mycobacterium leprae specific repetitive sequence in biopsy specimens from Leprosy patients. J Clin Microbiol 1993;31:895-9.
[Google Scholar]
|
| 12. |
Shin YC, Hyejon L, Hyeyoung L, Gerald PW, Deuk KJ, Nae CS. Variable numbers of TTC repeats in Mycobacterium leprae DNA from leprosy patients and use in strain differentiation. J Clin Microbiol 2000;38:4535-8.
[Google Scholar]
|
| 13. |
Singh N, Malik A, Aroara VK, Bhatia A. Fine needle aspiration cytology of leprous neuritis. Acta Cytol 2003;47:368-72.
[Google Scholar]
|
| 14. |
Banerjee S, Biswas N, Kanti Das N, Sil A, Ghosh P, Hasanoor Raja AH, et al. Diagnosing leprosy: Revisiting the role of the slit-skin smear with critical analysis of the applicability of polymerase chain reaction in diagnosis. Int J Dermatol 2011;50:1522-7.
[Google Scholar]
|
| 15. |
Chemouilli P, Woods S, Said G, Cole ST . Detection of Mycobacterium leprae in nerve lesions by the polymerase chain reaction. Int J Lepr Other Mycobact Dis 1996;64:1-5.
[Google Scholar]
|
| 16. |
Hazra B, Banerjee PP, Bhattacharyya NK, Gupta PN, Barbhunia JN, Sanyal S. Nerve biopsy in the diagnosis of leprosy. Indian J Dermatol 1997;42:174-7.
[Google Scholar]
|
Fulltext Views
3,513
PDF downloads
1,655





