Translate this page into:
Use of preoperative and perioperative ex vivo dermoscopy for precise mapping of margins for standard surgical excision of primary basal cell carcinoma
-
Received: ,
Accepted: ,
How to cite this article: Savant SS Jr. Use of pre-operative and perioperative ex vivo dermoscopy for precise mapping of margins for standard surgical excision of primary basal cell carcinoma. Indian J Dermatol Venereol Leprol 2023;89:793
Abstract
Background
The utility of preoperative and perioperative dermoscopy in standard surgical excision for radical excision of primary basal cell carcinoma remain unexplored.
Aims
To evaluate the use of preoperative and perioperative dermoscopy for precise mapping of margins during standard surgical excision of primary basal cell carcinoma.
Methods
In this retrospective, observational study, 17 patients clinically diagnosed with various morphological subtypes of basal cell carcinoma were included. Data about previous history, clinical examination of lesions and regional lymph nodes and preoperative dermoscopy were retrieved. After standard surgical excision had been carried out as per mapping of lateral margins, all the excised surgical specimens were subjected to perioperative dermoscopy and later reconfirmed with histopathology.
Results
Seventeen patients with mean age of 60.82 ± 9.99 years and median disease duration of 14 months were analysed. Clinically, basal cell carcinomas were of pigmented superficial subtype [6 (35.3%)], followed by pigmented nodular [5 (29.4%)], nodulo-ulcerative [4 (23.5%)] and micro nodular [2 (11.8%)]. Mean extension of clinical margin after dermoscopy was 0.59 ± 0.52 mm. Mean pre-assessed depth of tumour and mean depth of tumour were 3.46 ± 0.89 mm and 3.49 ± 0.92 mm, respectively. No recurrence was reported. Frequently found pre-operative dermoscopic features were maple leaf like structures [6 (35%)], blue grey dots and globules [6 (35%)] and short fine telangiectasias [6 (35%)]. Commonly observed perioperative dermoscopic features were: (1) irregular band with brown–grey pigmentation of dots, globules, streaks and pseudopodia like extensions [3 (50%)]; (2) irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with diffuse white streaks in pseudopodia like manner [1 (50%)]; (3) irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with streaks of white pseudopodia like structureless areas [1 (50%)].
Limitation
This was a single-centre study with a small sample size.
Conclusion
This study highlights significance of preoperative and perioperative dermoscopy for precise planning and radical excision of primary basal cell carcinoma by standard surgical excision.
Keywords
Basal cell carcinoma
dermoscopy
histopathology
Moh’s micrographic surgery
margins of excision
Plain Language Summary
Seventeen patients clinically diagnosed with morphological subtypes of basal cell carcinoma (BCC) located over different body sites were treated with standard surgical excision (SSE) followed by flap. A predetermined excisional margin was marked after mapping the lateral margin using pre-operative dermoscopy. The excised specimen where subjected to perioperative ex-vivo dermoscopy to confirm tumor free lateral and deep margins which was confirmed by post-operative histopathology. The study was started in September, 2015. No recurrence in any of the cases at 5 years follow-up. Thus, bringing out the importance of dermoscopy application in SSE of BCC where Moh’s micrographic surgery facility is not available.
Introduction
Dermoscopy is a noninvasive, diagnostic, imaging technique used for magnified illuminated visualisation of skin surfaces and subsurface structures of the epidermis and papillary dermis invisible to the naked eye for assessing various benign, premalignant and malignant skin lesions including basal cell carcinoma with high accuracy. 1,2 Basal cell carcinoma is a malignant epidermal skin cancer usually affecting sun-exposed area in white–skin populations and consists of various morphological subtypes with each having its characteristic clinical, histological and dermoscopic features. 3–5 Primary (not treated earlier) basal cell carcinoma is a slow-growing tumour with high recurrence rate, causing local infiltration, destruction and cosmetic anomaly warranting local intervention. 6
This study highlights the novel use of pre- and intra-operative dermoscopy in lesional and peri-lesional skin through vertical and horizontal sections to meticulously demarcate the peripheral border, margin and base to excise basal cell carcinoma (BCC) and correlate them histopathologically for optimizing and concising Moh’s micrographic surgery. Dermoscope guided excisional flap surgery can not only be time saving and cost effective but easily be adapted by dermato-surgeons to achieve excellent aesthetic and oncologic outcomes with good functional restoration to prevent untoward sequelae of recurrence or metastasis.
Moh’s micrographic surgery, with 100% perioperative histological control is the treatment of choice as it has the least recurrence rate (<2%) and preserves the adjacent healthy tissue. 6–9 However, standard surgical excision, with predetermined excision margin varying between 3 and 10 mm according to site, size, previous treatment, histopathology, etc., remains the gold standard for radical excision as it has low recurrence rate (1.5–10.1%). 3,6,9 In both Moh’s micrographic surgery and standard surgical excision, the surgical defect is repaired either by primary closure or graft or flap or allowing it to heal by secondary intention. 3,6,7,9 While in Moh’s micrographic surgery surgical margins (lateral and deep) are taken care of perioperatively, in standard surgical excision lateral surgical margins need to be defined preoperatively by proper markings through pre-operative dermoscopy and deep margins to be taken care of by carrying out excision through subcutaneous fat in order to avoid chances of recurrence. 6,9 The preoperative dermoscopy is useful to determine the lateral margin for accurately detecting the contiguous extension of the tumour; however, it cannot assess the deep margin; hence, there can be recurrence. 1,2,10 Presently, Moh’s micrographic surgery is the only procedure that can map both lateral and deep margins through histopathology but is not available everywhere and hence standard surgical excision is the next best commonly followed surgical option for complete removal of primary basal cell carcinoma. 6–9
In this study, micrographic control was achieved by combining preoperative (lesional, perilesional, radial and peripheral border) and intraoperative (lesional, perilesional, base, vertical and horizontal–vertically bisected excised specimen) dermoscopy for accurately gauging the tumoural depth along with its contiguous deep and lateral extension. The detection of deep and lateral extension, if found close to the dermal subcutaneous basal margin or lateral margins on perioperative dermoscopy, enables early reintervention by additional surgical excision, as in Moh’s micrographic surgery, thus, reducing the chances of recurrence. Dermoscopic findings were later confirmed histologically. The interesting part of the study is the use of preoperative and perioperative dermoscopy in standard surgical excision for radical excision of primary basal cell carcinoma, which is not well known.
Method
Study population
This was a retrospective, observational study conducted at The Humanitarian Clinic: Skin, Hair and Laser Centre, Mumbai, India from September, 2015 till December, 2020. Ethical approval was taken from Institutional Ethics Committee (Registration number: ISBEC/NR-2/KM-KM/2022) and the study was conducted in accordance with Declaration of Helsinki. Written informed consent was already taken from all the patients. Patients having lesions that were clinically compatible with the morphological subtypes of primary basal cell carcinoma were included in the study.
Exclusion criteria
Basal cell carcinoma that was recurrent, morpheaform, superficial multifocal, locally advanced—more than 10 cm in size with enlarged regional or distant lymph nodes/metastasis, and those which showed positivity on positron emission tomography scan studies, were excluded from the study.
Preoperative workup
History (medical/surgical, past treatment), clinical examination of the lesion (morphological subtype, anatomical location, size) and regional lymph nodes were recorded as per a predefined proforma.
Preoperative dermoscopy
The clinical margin of the lesion was marked. Non-polarised, noncontact surface dermoscopy was carried out with medi-cam 800HD, D-scope 2 plus lens with 20 X to 120 X magnification (Foto Finder, Germany–Geomedics, Pune, India).
Lesional and perilesional: The dermoscopic features were observed in all the clinical subtypes to correlate with the final histological diagnosis [Table 1]. In the patients with superficial basal cell carcinoma, the dermoscopic margin was marked [Figure 1]. It was either overlapping with the marked clinical margin and or extended over the clinical margin by 0.5–1.5 mm (11 cases). The outermost combined margin of these two was considered as clinico-dermoscopic margin. As per the planned flap, a final surgical margin was then marked encompassing this clinicodermoscopic margin [Figures 2 and 3]. It varied from 3 to 5 mm (mean of 4.5–5 mm) for lesions measuring ≤2 cm and 5–10 mm (mean of 7.5 mm) for lesions measuring ≥2 cm along maximum diameter, respectively. This was further fortified to suit the designed flap taking various local factors such as anatomical location, relaxed skin tension lines, line of maximum extensibility, etc.
| Characteristics | N (%) |
|---|---|
| Mean age, years | 60.8 ± 10.0 |
| Male and Female | 11 (64.7) |
| Mean duration of disease, months | 15.7 ± 7.9 |
| Anatomical location of basal cell carcinoma | |
| Back | 1 (5.9) |
| Cheek | 6 (35.3) |
| Preauricular area | 2 (11.8) |
| Neck | 3 (17.6) |
| Parasternal area | 1 (5.9) |
| Lateral side of trunk | 1 (5.9) |
| Mid-line of forehead | 1 (5.9) |
| Temporal area of forehead | 2 (11.8) |
| Clinical subtypes of basal cell carcinoma | |
| Nodulo-ulcerative | 4 (23.5) |
| Pigmented nodular | 5 (29.4) |
| Pigmented superficial | 6 (35.3) |
| Micronodular | 2 (11.8) |
| Mean extension of clinical margin, mm | 0.59 ± 0.52 |
| Mean size of tumour, mm | 3.49 ± 0.92 |
| Mean pre-assessed depth of tumour, mm | 3.46 ± 0.89 |
| Treatment procedure | |
| Bilobed flap | 4 (23.5) |
| Burrow’s wedge advancement flap | 1 (5.9) |
| Double hatchet flap | 2 (11.8) |
| Jigsaw puzzle flap | 1 (5.9) |
| Reading mans flap | 1 (5.9) |
| Rhomboid flap | 5 (29.4) |
| Rhomboid flap with 2 z plasties | 3 (17.6) |
Data are presented as n (%) or mean ± SD, SD: standard deviation
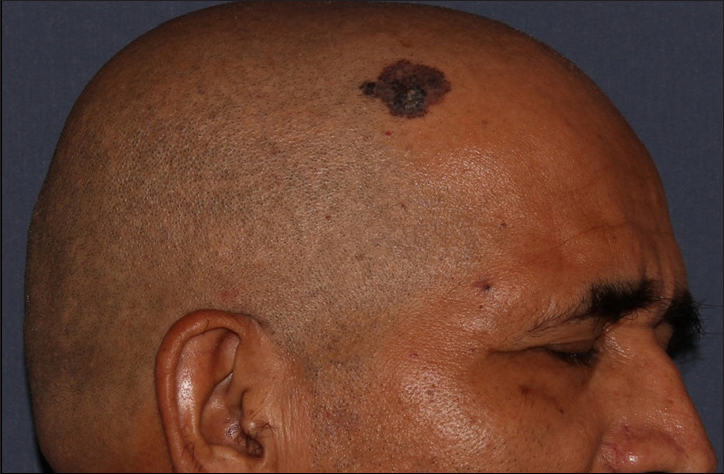
- Superficial pigmented basal cell carcinoma
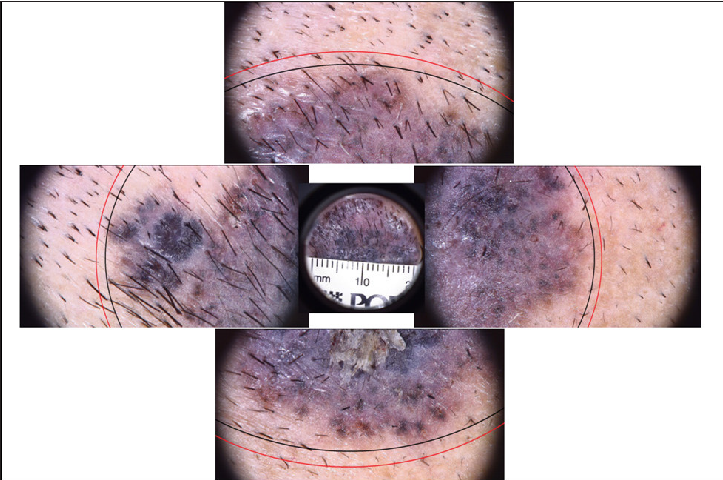
- Superficial pigmented basal cell carcinoma pre-operative dermoscopy with clinical marking (in black ink) & dermoscopic marking (in red ink)
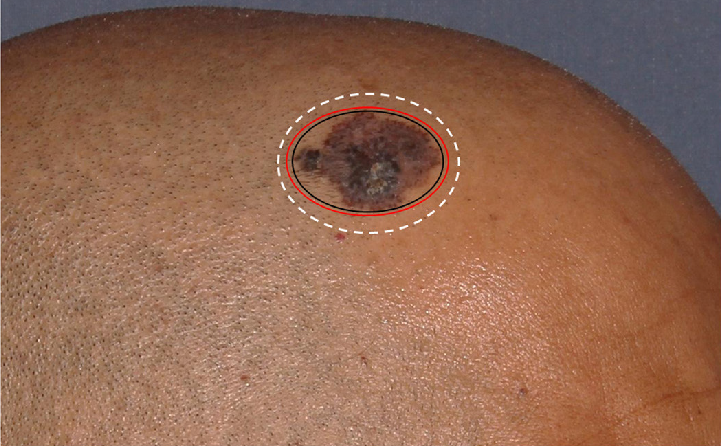
- Superficial pigmented basal cell carcinoma pre-operative dermoscopy with clinico-dermoscopic marking (in black & red ink) and surgical marking (in white ink)
Radial: Dermoscope was moved from the centre towards marked surgical margin in a slow radial manner clockwise over the entire lesion for observing any residual tumour or its extension from the clinico-dermoscopic margin.
Peripheral: Dermoscopy was carried out by going all along the marked peripheral surgical margin to observe for any tumoural extension.
Surgical procedure
Part was surgically prepared and locally anaesthetised with 6 mL of 2% lignocaine with adrenaline and 6 mL of 0.5% bupivacaine diluted in 18 mL of distilled water. Primary incision was taken along the marked surgical margin through the subcutaneous fat to excise the specimen and the defect was covered with moist gauze [Figure 4]. This allows for additional surgical excision of lateral or deep margins as in Moh’s micrographic surgery during surgery, if required, depending upon the perioperative dermoscopic findings.
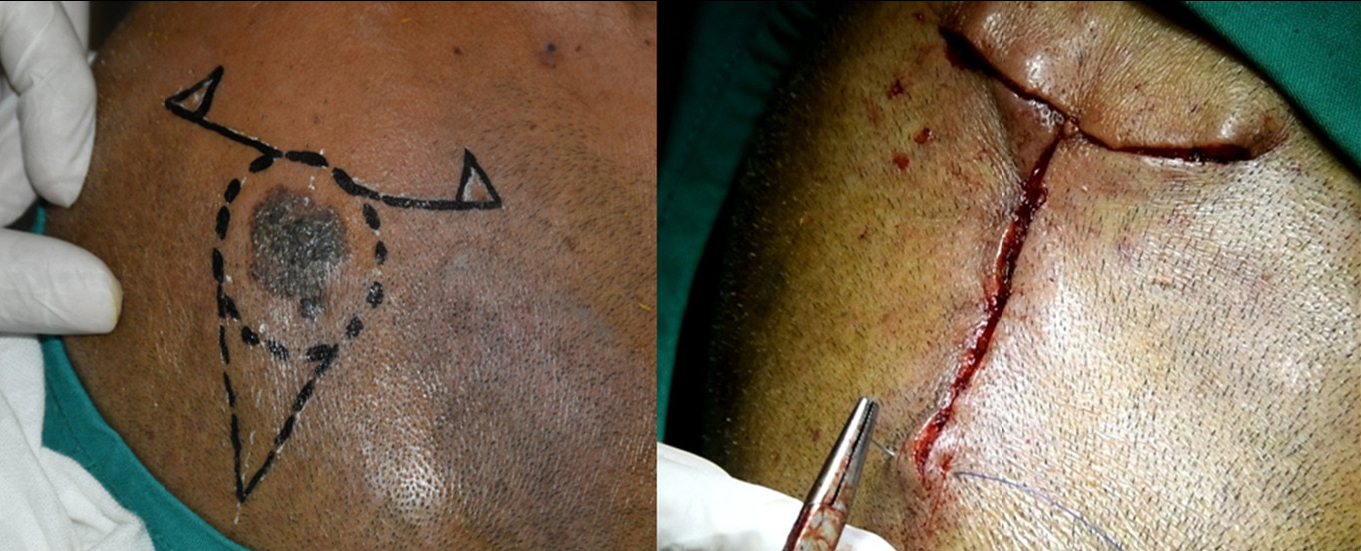
- Superficial pigmented basal cell carcinoma intra-operative
Perioperative dermoscopy
Excised specimen was cleansed with normal saline and dermoscopy was carried out ex vivo. [Figure 5].
Lesional and perilesional: The lens of the dermoscope was placed at the centre of the excised primary defect perpendicular to the epidermal surface followed by the periphery over the perilesional normal appearing border and surgical margins going in a slow radial centrifugal manner to observe for any residual tumour or its extension towards the margin [Figure 5].
Base: The excised specimen was flipped over to place its base grossly comprising of subcutaneous fat facing upwards and epidermal surface downwards [Figure 5]. Non-contact surface dermoscopy was carried out from the centre to the periphery in slow radial clockwise manner to observe for any residual tumour extensions.
Direct (X and Y axis): The specimen was oriented horizontally and bisected vertically along its maximum diameter dividing it into two equal parts with sharp razor blade to avoid distortion [Figure 6]. The two specimens were laid on their sides and the dermoscope was moved in non-contact way over all the peripheral margins, border and base vertically in Y axis from epidermal surface towards the subcutaneous fat as well as horizontally in X axis in the dermal plane from one end to another, thus covering the entire specimen to obtain three-dimensional images [Figures 7 and 8] to scrutinise any unrecognised subclinical deep and lateral extensions and to ensure that complete radical excision of the basal cell carcinoma has been achieved. It aided in conjecturing the depth and extent of tumour along with the thickness of tumour-free dermal subcutaneous tissue at the base. It also acted as a guide in determining the peripheral tumour-free epidermal, dermal and subcutaneous tissue at the margins in each case. The excised specimen was then sent for histological study to correlate and confirm the preoperative and perioperative dermoscopic findings [Figure 9]. The wound was then closed in two layers [Figure 10]. None of the patients required additional surgical resection as the perioperative dermoscopy did not reveal any involvement of deep or lateral margins.
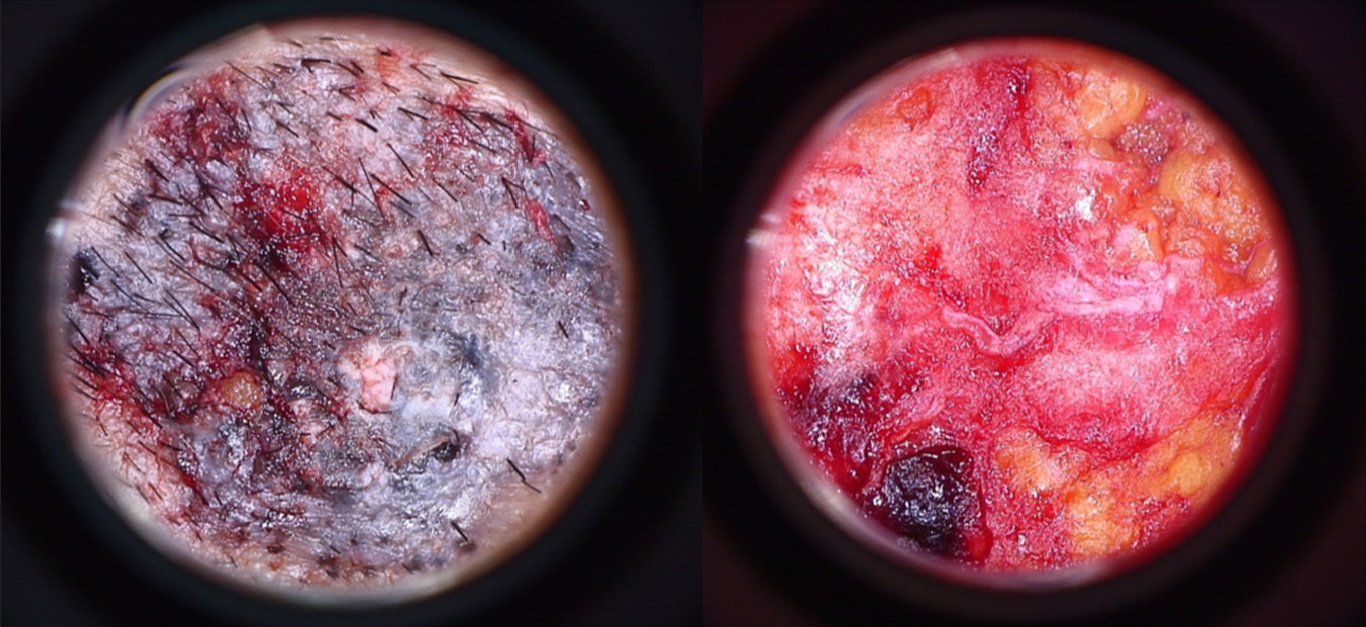
- Superficial pigmented basal cell carcinoma ex-vivo dermoscopy of surface (left) and base (right)
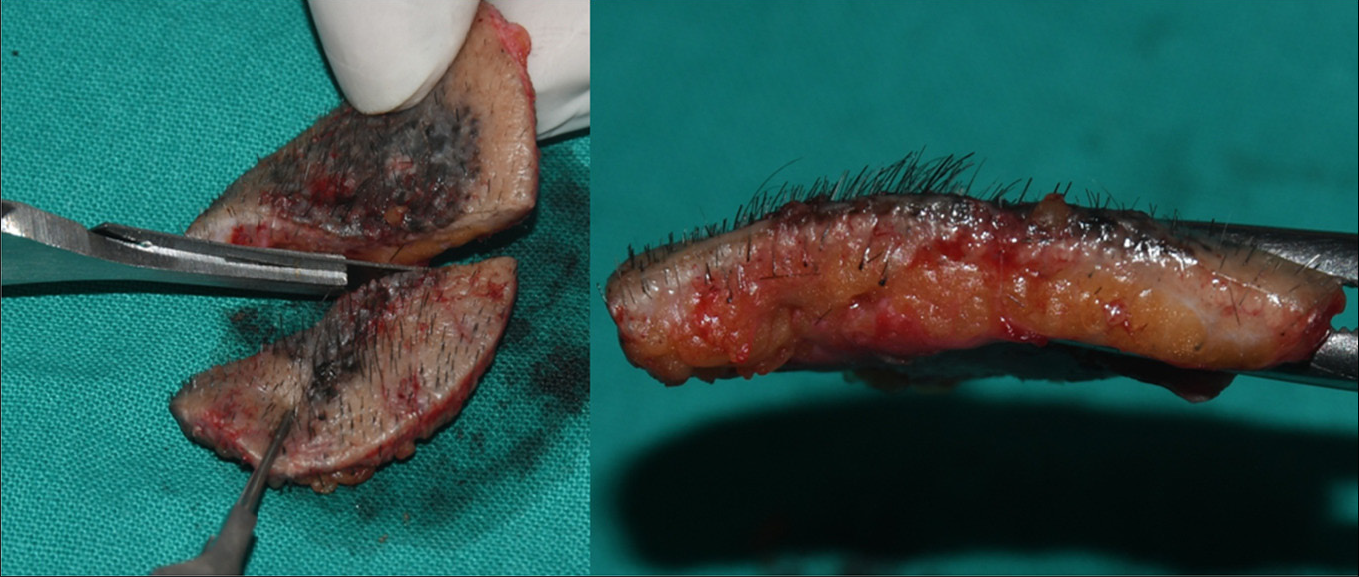
- Superficial pigmented basal cell carcinoma intra-operative vertical section with sharp razor blade
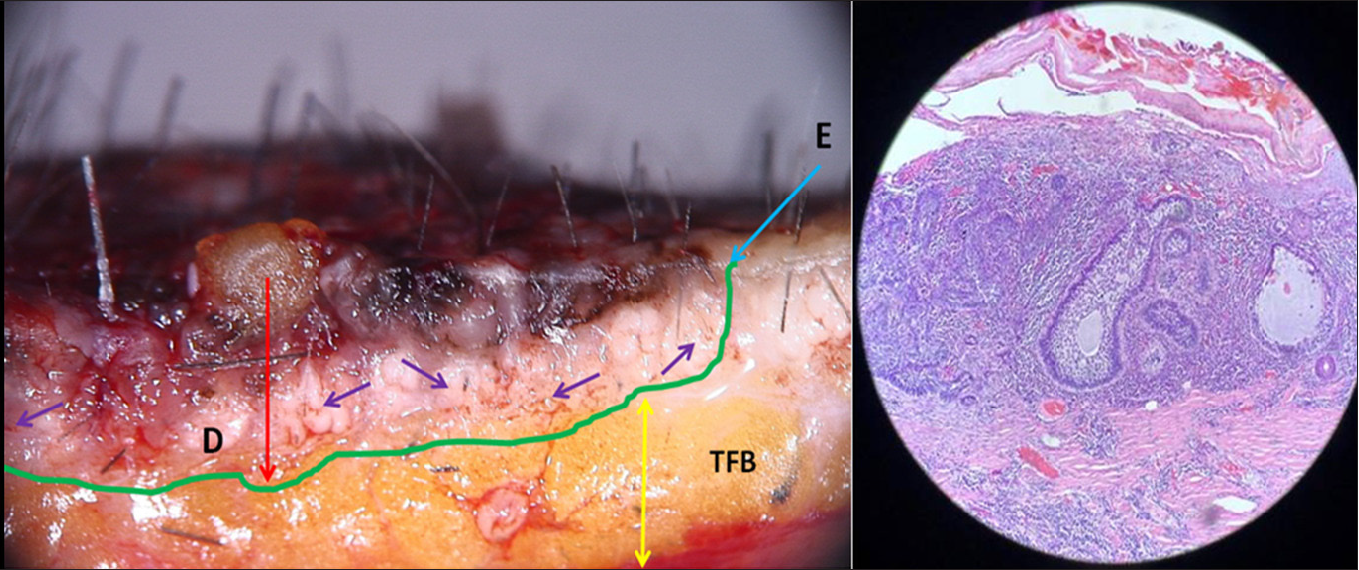
- Superficial pigmented BCC intra operative dermoscopy of vertical section; blue arrow indicates extension of lesion, red arrow indicates depth of tumour, purple arrow indicates brown globules of basaloid cells, and yellow arrow indicates tumour free border
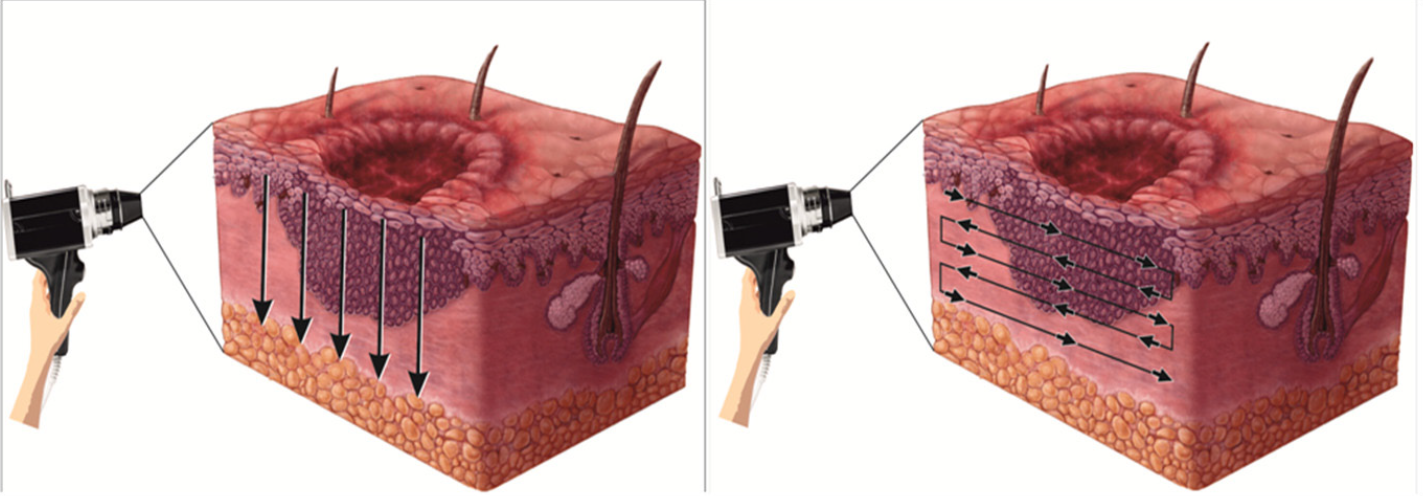
- Intra operative direct dermoscopy movement indicated by vertical arrows pointing downwards in y-axis and horizontal arrows pointing side to side in x-axis for three-dimensional view of peripheral margin, border and base in epidermal-dermal plane
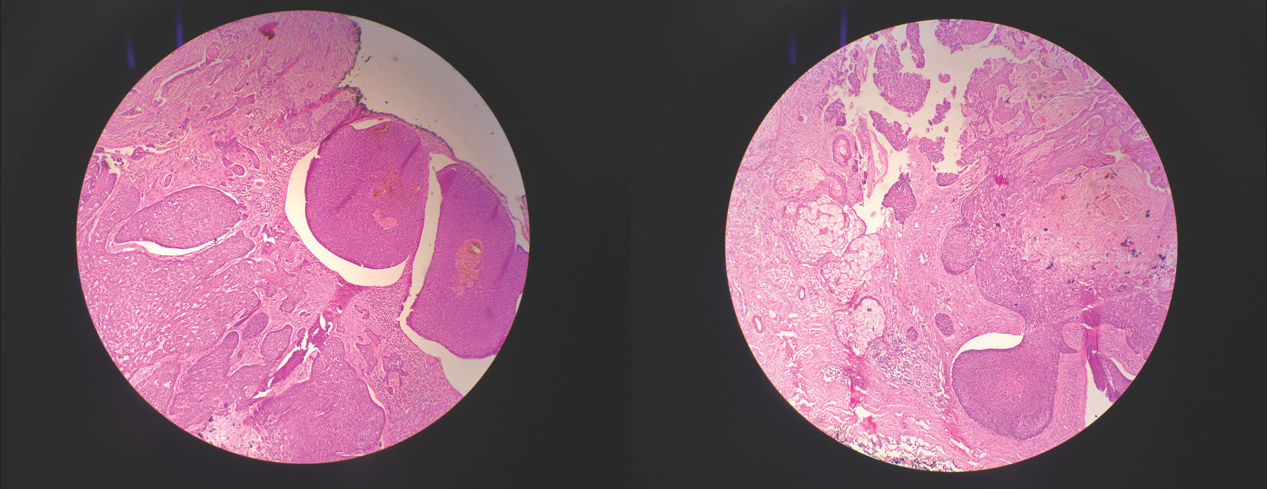
- (a) Histopathology 20x magnification, bulbous extension of pigmented nest of basaloid cells having common base at periphery of tumour at level of dermo-epidermal junction and in superficial papillary dermis, (b) Histopathology 20x magnification, large nests of basaloid cells with pigment aggregates invading into the dermis
Postoperative workup
Postoperatively wound was dressed with antibiotic ointment. Broad-spectrum antibiotics and analgesic/anti-inflammatory (as and when required) were given for 10 days. The sutures were removed after 10–15 days. All the lesions healed by 6 months with fine linear scar [Figure 10].
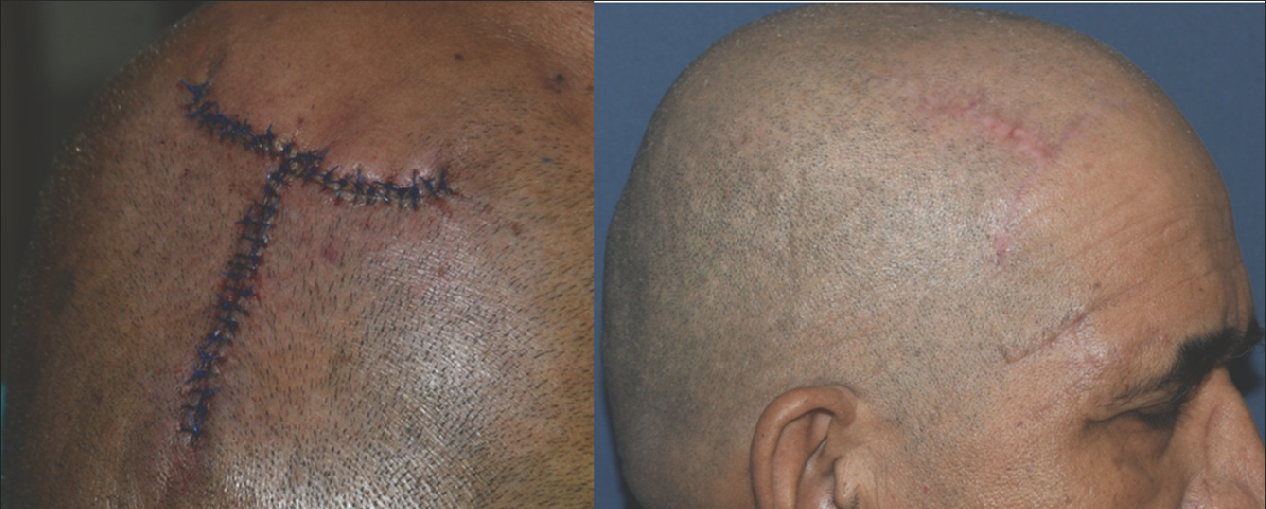
- Superficial pigmented basal cell carcinoma primary closure with simple interrupted sutures (left) and post operative after 6 months (right)
Follow-up
All patients were initially followed up once a month for 3 months and then once in 3 months for next 1 year, and then every 6 months up to 5 years.
Results
Mean pre-assessed depth of tumour and mean depth of tumour were 3.46 ± 0.89 mm and 3.49 ± 0.92 mm, respectively. Mean extension of the clinical margin after dermoscopy was 0.59 ± 0.52 mm. Clinically, the majority of basal cell carcinomas were of the pigmented superficial subtype [6 (35.3%), followed by pigmented nodular [5 (29.4%)], nodulo-ulcerative [4 (23.5%)] and micronodular [2 (11.8%)] [Table 1]. Clinical, dermoscopic (pre and intra) and histopathology [Figure 10] details of all cases are summarised in Table 2. Base was uninvolved and margins were tumour free in all the excised specimen histopathologically. On pre-operative dermoscopic findings, maple leaf like structures [6 (35%)], blue grey dots and globules [6 (35%)] and short fine telangiectasias [6 (35%)] were found to be the most frequent dermoscopic features [Table 3]. As shown in Table 4, the most commonly observed dermoscopic features were: (1) irregular band with brown–grey pigmentation of dots, globules, streaks and pseudopodia like extensions [3 (50%)]; (2) irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with diffuse white streaks in pseudopodia like manner [1 (50%)]; (3) irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with streaks of white pseudopodia like structureless areas [1 (50%)]. All the lesions healed completely with good functional, oncological and aesthetic outcomes without recurrence on follow-up of 5 years.
No.
Clinical type
Pre-assessed depth of tumour (mm)
Histopathology
Observational error in depth of tumour (mm)
1
Nodulo-ulcerative
4.8
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 4.9 mm; margin and base uninvolved by tumour
+0.1
2
Pigmented nodular
3.3
Nodules of basaloid cells with scant cytoplasm and hyperchromatic nuclie with peripheral palisadings; abundant melanin pigment in stroma; tumour depth 3.3 mm; margin and base uninvolved by tumour
0
3
Pigmented nodular
3.9
Nodules of basaloid cells with scant cytoplasm and hyperchromatic nuclie with peripheral palisadings; abundant melanin pigment in stroma; tumour depth 3.9 mm; Margin and base uninvolved by tumour
0
4
Pigmented superficial
2.9
Sheets of basaloid cells showing peripheral palisading of nuclei in upper dermis; sparse lymphocytic infiltrate at base of tumour; tumour depth 3 mm; margin and base uninvolved by tumour
+0.1
5
Pigmented superficial
3
Sheets of basaloid cells showing peripheral palisading of nuclei in upper dermis; sparse lymphocytic infiltrate at base of tumour; tumour depth 2.9 mm; margin and base uninvolved by tumour
−0.1
6
Pigmented superficial
3.1
Sheets of basaloid cells showing peripheral palisading of nuclei in upper dermis; sparse lymphocytic infiltrate at base of tumour; tumour depth 3.1 mm; margin and base uninvolved by tumour
0
7
Pigmented superficial
2.5
Sheets of basaloid cells showing peripheral palisading of nuclei in upper dermis; sparse lymphocytic infiltrate at base of tumour; tumour depth 2.5 mm; margin and base uninvolved by tumour
0
8
Pigmented nodular
2.1
Nodules of basaloid cells with scant cytoplasm and hyperchromatic nuclie with peripheral palisadings; abundant melanin pigment in stroma; tumour depth 2.1 mm; margin and base uninvolved by tumour
0
9
Nodulo-ulcerative
2.2
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 2.4 mm; margin and base uninvolved by tumour
+0.2
10
Pigmented nodular
2.6
Nodules of basaloid cells with scant cytoplasm and hyperchromatic nuclie with peripheral palisading; abundant melanin pigment in stroma; lymphoplasmacytic infiltrate; tumour depth 2.5 mm; margin and base uninvolved by tumour
−0.1
11
Pigmented superficial
3.4
Neoplasm with nest of basaloid cells with peripheral palisading of nuclei in upper dermis; abundant melanin and lymphocytic infiltrate; tumour depth 3.4 mm; margin and base uninvolved by tumour
0
12
Pigmented nodular
4.2
Nodules of basaloid cells with scant cytoplasm and hyperchromatic nuclie with peripheral palisadings; melanin pigment in stroma; tumour depth 4.1 mm; margin and base uninvolved by tumour
−0.1
13
Nodulo-ulcerative
4.2
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 4.2 mm; margin and base uninvolved by tumour
0
14
Nodulo-ulcerative
3.7
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 3.8 mm; margin and base uninvolved by tumour
+0.1
15
Pigmented superficial
3.3
Neoplasm with nest of basaloid cells with peripheral palisading of nuclei in upper dermis; abundant melanin and lymphocytic infiltrate; tumour depth 3.3 mm; margin and base uninvolved by tumour
0
16
Micronodular
4.7
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 4.7 mm; margin and base uninvolved by tumour
0
17
Micronodular
5
Tumour composed of infiltrating nests and islands of basaloid cells with darkly stained nuclei and peripheral palisading; nuclear and mitoses; ulceration; dense inflammation; tumour depth 5.2 mm; margin and base uninvolved by tumour
+0.2
Dermoscopic findings
N (%)
Nodulo-ulcerative (4)
Peripheral arborising vessels
4 (100%)
Keratin masses
4 (100%)
Ulceration
4 (100%)
Pigmented nodular (5)
Arborizing telangiectasias
4 (100%)
Ulceration
4 (100%)
Blue grey ovoid nest
5 (100%)
Branching arborising vessels
1 (100%)
Pigmented superficial (6)
Maple leaf like structures
6 (100%)
Blue grey dots and globules
6 (100%)
Short fine telangiectasias
6 (100%)
Micronodular (2)
Peripheral arborising vessels
2 (100%)
Keratin masses
2 (100%)
Ulceration
2 (100%)
Dermoscopic findings
N (%)
Nodulo-ulcerative (6)
Irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with dots and globules
2 (33.3%)
Irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with few diffuse areas of light brown dots and globules
1 (16.7%)
Irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with few diffuse areas of dots and globules
1 (16.7%)
Pigmented nodular (5)
Irregular band in psoriasiform pattern of dark brown-black pigmentation with dots, globules, streaks and structureless areas
2 (40.0%)
Irregular band of dark brown-black pigmentation with dots, globules, structureless areas and increased vascularity
1(20.0%)
Irregular band of dark brown-black pigmentation with dots, globules, structureless areas and increased number of blood vessels
1 (20.0%)
Irregular band of brown-black pigmentation with dots, globules, structureless areas and few pseudo granulomatous areas of increased vascularity
1 (20.0%)
Pigmented superficial (6)
Irregular band with brown-grey pigmentation of dots, globules, streaks and pseudopodia like extensions
3(50%)
Irregular band with ochre brown pigmentation of dots, globules, streaks and increased vascularity
2 (33.3%)
Irregular diffuse band with light brown pigmentation of dots, globules and streaks
1 (16.7%)
Micronodular (2)
Irregular band of pseudo granulomatous structureless vascular areas in psoriasiform pattern with diffuse white streaks in pseudopodia like manner
1 (50%)
Irregular band of pseudo granulomatous structureless vascular areas in psoriasis form pattern with streaks of white pseudopodia like structureless areas
1 (50%)
Discussion
Dermoscopy improves diagnostic accuracy by correlating and confirming the clinical and histopathological subtypes of basal cell carcinoma. 1,2,5,11 Reiter et al. 12 reported its sensitivity as 91.2% and specificity as 95% for diagnosis of basal cell carcinoma. In this study of 17 basal cell carcinoma patients, Preoperative dermoscopy was useful in correlating the clinical subtypes with the final histopathological diagnosis in all the cases.
A previous report by Álvarez et al. 13 demonstrated that blue-gray dots and globules (27%) and maple leaf-like areas (25%) as the most common dermoscopic structures in superficial pigmented basal cell carcinoma lesions. Tabanlıoğlu et al. 14 showed spoke-wheel areas, large blue-grey ovoid nests and multiple blue-grey globules as the most common dermascopic structures for superficial pigmented basal cell carcinoma. Only a few studies have specifically explored the dermoscopic features of micronodular basal cell carcinoma, but there have been reports of truncated vessels and multiple blue-grey globules. 15,16 Dermoscopic examination of pigmented nodular basal cell carcinoma typically shows blue-grey ovoid nests (36%), usually in combination with arborising telangiectasias. 17,18 Less common pigmented structures are blue-grey globules and dots. 19 In the present study, maple leaf like structures, blue grey dots and globules and short fine telangiectasias were highly predictive of pigmented superficial basal cell carcinoma. The remaining features according to clinical subtypes are outlined in Table 3. To the best of our knowledge, no studies have explored intraoperative dermoscopic findings of basal cell carcinoma in such a concise manner.
It is difficult to clinically detect the malignant extensions of basal cell carcinoma due to the contiguous microscopic nature of its spread. 3,6,7 Caresana and Gardini 20 used dermoscopy preoperatively in 200 cases of basal cell carcinoma to determine the peripheral border. They reported overlapping of clinical and dermoscopic margins in 131 (65.5%) and peripheral extension of clinical margin in 69 (34.5%) cases. Reiter et al. 12 reported that adding dermoscopy to naked eye examination improved the sensitivity from 66.9% to 85% (P = 0.0001) and specificity from 97.2% to 98.2% (P = 0.006). Suzuki et al. 10 reported the extension of clinical margin by 0.5–2.26 mm in all (100%) cases in the intervention group. In this study, the clinical margin was extended by 0.5–1.5 mm after preoperative dermoscopy in 11 (64.7%) out of 17 cases. In standard surgical excision, it is advocated that a predetermined surgical margin (3–10 mm), as per the standard protocol, should be left around the tumour taking into consideration the microscopic tumoural spread. 6,7,9,21 Wolf and Zitelli et al. 21 reported that 4 mm margins cleared 98% of basal cell carcinomas ≤ 2 cms. in diameter. Kumar et al. 22 have reported incomplete excision of 4.2%, 4.1%, and 2.9% in 757 cases of basal cell carcinomas excised with margins of 1–2.5, 3–4 and 5 mm or more, respectively. Caresana and Gardini. 20 extended the clinico-dermoscopic border by 2 mm in 200 cases to achieve histologically confirmed complete excision with 98.5% cure rate. In the present study, the clinico-dermoscopic margin was extended by 3–5 and 6–10 mm for the basal cell carcinomas measuring <2 cm and >2 cm, respectively and a histologically confirmed complete excision with 100% cure rate was achieved.
Griffiths et al. 23 have reported that the depth of basal cell carcinoma ranges from 0.1 to 9.9 mm and the lesion should be excised accordingly to get clearance of its deep margin. In this study, during perioperative dermoscopy, the depth of basal cell carcinoma varied from 2.1 to 5.0 mm, nearly matching the postsurgical histological depth of 2.1–5.2 mm and no tumour extension was noted in the subcutaneous fat at the base of the specimen. The standard protocol recommends that the incision should be wide and through subcutaneous fat for complete removal and to avoid recurrence. 6,7,9,21 This was confirmed by us in all the 17 cases. Incomplete excision usually recurs in 4–12 months and the recurred lesions are more aggressive requiring further wider surgical excision. 6,7,9,21,22,24,25 5 years’ recurrence rate after Moh’s micrographic surgery for primary basal cell carcinoma is ˂2% whereas it varies from 1.5 to 10.1% with standard surgical excision. 6–9,20,21,25,26 We did not observe any recurrence in any of the cases on follow-up of 5 years.
Among the various modalities, Moh’s micrographic surgery or standard surgical excision are recommended for treating primary basal cell carcinoma, where the excised tissue is available for either intraoperative histology (Moh’s micrographic surgery) or postoperatively (standard surgical excision) for confirmation of tumour clearance, which is not so with other modalities such as cryosurgery, curettage, radiotherapy and photodynamic therapy. 6 Moh’s micrographic surgery is the most effective as it accurately detects and treats the contiguous growth spread. 6,8,9 Moh’s micrographic surgery is a multi-staged procedure in which the lesion with 1–2 mm of the peripheral margin is excised and subjected through a tedious process (orientation, dissection, colour coding, freezing in liquid nitrogen, moulding and sectioning) to obtain horizontal frozen section for microscopic examination. 6,8–10,26 Multiple serial excisional steps are repeated till complete tumour excision is microscopically confirmed while the patient and surgical team are waiting in the operation theatre. This is a time-consuming, laborious, expensive method that requires Moh’s laboratory, trained surgeon and experienced histological expertise. 6,27–29 In India, Moh’s micrographic surgery is available at a few places only under limited resources. Hence, wherever access to Moh’s micrographic surgery facility is not available, standard surgical excision as a one stage procedure is most commonly practised for treating primary basal cell carcinoma. 6,7,9,28,29 In this study, preoperative dermoscopy was combined with perioperative dermoscopy to achieve an almost equivalent microscopic control as in Moh’s micrographic surgery. In this study, good oncosurgical and aesthetic results without any functional disturbance were achieved through precise planning of a flap suitable to cover the surgical defect. Radical excision of the primary basal cell carcinoma was achieved cost effectively by innovatively combining preoperative and perioperative dermoscopy, with 100% cure rate and no recurrence at 5-year follow-up. The preoperative dermoscopy could confirm the clinical diagnosis and help in reaching a presumptive histological diagnosis of the subtypes. It further guided in prediction of the peripheral spread of the tumour. The perioperative dermoscopy helped in accurately detecting tumoural depth and gauging the deep basal and lateral peripheral spread. This further provides the operator guidelines to undertake any additional surgical excision similar to Moh’s micrographic surgery if required. This detailed planning aided in accomplishing a complete comprehensive diagnostic and therapeutic radical excision to achieve histologically confirmed tumour-free plane in one stage, with good oncosurgical and aesthetic results without any functional disturbance. We believe, in future, with advancement in the field of dermoscopy and its adaptation by dermatosurgeons, standard surgical excision may replace Moh’s micrographic surgery.
Limitation
The study is limited by retrospective nature of the study. Moreover, a single-center design comprising a small sample size is another limitation.
Conclusion
We have demonstrated the importance of preoperative and perioperative ex vivo dermoscopy for precise planning of standard surgical excision in primary basal cell carcinoma. It is a simple, safe, inexpensive and excellent method to determine the lateral margin to assist in marking the final surgical incision lines and to visualize the lateral along with deep tumoural margins especially with high magnification of 120X to get a three-dimensional view.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Research techniques made simple: Non-invasive imaging technologies for the delineation of basal cell carcinomas. J Invest Dermatol. 2016;136:e33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in basal cell carcinoma: An update review. Actas Dermosifiliogr. 2021;112:330-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tumours and cysts of the epidermis. In: Williams Williams, El-Der DE, eds. Lever’s Histopathology of the Skin (10th ed). Philadelphia, PA: Wolters Kluwer/Lippincott; 2008. p. :791-850.
- [Google Scholar]
- Dermoscopy of basal cell carcinoma. Clin Exp Dermatol. 2018;43:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma: 10 years of experience. J Skin Cancer. 2011;2011:476362.
- [CrossRef] [PubMed] [Google Scholar]
- British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48.
- [CrossRef] [PubMed] [Google Scholar]
- Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-31.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of basal cell carcinoma: An algorithm based on the literature. An Bras Dermatol. 2015;90:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopy of basal cell carcinoma: Morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systemic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in basal cell carcinoma: An updated review. Actas Dermosifiliogr (Engl Ed). 2021;112:330-8.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between the dermatoscopic and histopathological features of pigmented basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1317-25.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of dermatoscopy for the preoperative assessment of the histopathologic aggressiveness of basal cell carcinoma. Ann Dermatol. 2015;27:682-7.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of dermoscopic findings with histopathologic variants of basal cell carcinoma. Int J Dermatol. 2013;52:718-21.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopic features of basal cell carcinoma and its subtypes: A systematic review. J Am Acad Dermatol. 2021;85:653-664.
- [CrossRef] [PubMed] [Google Scholar]
- A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: Effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- [CrossRef] [PubMed] [Google Scholar]
- The dermatoscopic universe of basal cell carcinoma. Dermatol Pract Concept. 2014;4:11-24.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2010;24:1395-9.
- [CrossRef] [PubMed] [Google Scholar]
- Incomplete excision of basal cell carcinoma: A prospective multicentre audit. Br J Plast Surg. 2002;55:616-22.
- [CrossRef] [PubMed] [Google Scholar]
- Basal cell carcinoma histological clearance margins: An analysis of 1539 conventionally excised tumours. Wider still and deeper? J Plast Reconstr Aesthet Surg. 2007;60:41-7.
- [CrossRef] [PubMed] [Google Scholar]
- Histological evolution of recurrent basal cell carcinoma and therapeutic implications for incompletely excised lesions. Br J Dermatol. 2004;151:623-6.
- [CrossRef] [PubMed] [Google Scholar]
- Long–term recurrence rates in previously untreated primary basal cell carcinoma: Implications for patients follow up. J Dermatol Surg Oncol. 1989;15:315-28.
- [CrossRef] [PubMed] [Google Scholar]
- Mohs micrographic surgery for the treatment of basal cell carcinoma. Actas Dermosifiliogr. 2010;101:853-7.
- [PubMed] [Google Scholar]
- Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: A prospective randomized controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of face: A randomised controlled trial. Lancet. 2004;50:3011-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cost effectiveness of surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma. Arch Dermatol. 2006;142:187-94.
- [CrossRef] [PubMed] [Google Scholar]






