Translate this page into:
Utility of a real-time fluorescence imaging device in guiding antibiotic treatment in superficial skin infections
Corresponding author: Dr. Aayush Gupta, Department of Dermatology, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pimpri, Pune, Maharashtra, India. aayushggupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shah BM, Ganvir D, Sharma YK, Mirza SB, Misra RN, Kothari P, et al. Utility of a real-time fluorescence imaging device in guiding antibiotic treatment in superficial skin infections. Indian J Dermatol Venereol Leprol 2022;88:509-14.
Abstract
The prescription of antibiotics empirically without confirmation of an infective etiology is on the rise. Administration of appropriate antibiotics can be guided by real-time fluorescence imaging using a point-of-care device. These composite images show the presence, type and the burden of infection. The time saved by this method over microbiological testing, especially in resource-poor settings, can lead to a paradigm shift in treatment by facilitating prompt and adequate antimicrobial therapy, surgical debridement as well as follow-up. Thumbnail sketches of a series of four cases highlighting different scenarios in which a fluorescent imaging device utilizing artificial intelligence and machine learning was found useful is presented in this report.
Keywords
Antimicrobial stewardship
fluorescence imaging
Illuminate
infections
wound care
Introduction
Most of the skin and soft tissue infections resolve with treatment, however, some worsen forming chronic ulcers that impair not only the patients’ quality of life but strain an already overburdened healthcare system. An escalating incidence of secondarily infected chronic wounds has led to increased hospitalization of patients as well as antibiotic usage.1
The incidence of acute and chronic wounds in India is 10.5 and 4.5 per 1000 population respectively.2 Secondary bacterial infections commonly complicate the healing of diabetic ulcers leading to the loss of a lower limb every 30 seconds.3 Initial clinical evaluation and microbiological correlation of an infected wound typically takes up to 10 days. An already tedious recovery may get prolonged by incorrect therapy consequent to a cursory examination, pending culture and sensitivity results, or inaccurate reports following improper swabbing. Moreover, despite proper swabbing/aseptic techniques, all organisms may not be isolated especially in comparison with that of deep tissue biopsies (current gold standard).4,5
We used a novel handheld imaging device, “Illuminate,” developed by Adiuvo Diagnostics (Chennai, India), which aims to accelerate the treatment of skin infections by rectifying some of the above-mentioned lacunae. This device leverages autofluorescence, artificial intelligence and machine learning to accurately classify/locate the microorganisms and provide semiquantitative data regarding the infective burden of the wound. Pointed to the region of interest, the device captures 16 serial images at different excitation emissions and produces a clinical image overlaid with the superimposed infection, if any, marking the presence of gram-positive bacteria in red, gram-negative in green, and fungus in blue. Four cases where the device was found invaluable are summarized below.
Case Reports
Case 1
A 73-year-old female had multiple and painful non-healing recalcitrant ulcers on both thighs [Figure 1a] for 9 months, despite multiple courses of tablet amoxicillin/clavulanic acid, tablet azithromycin, and injectable amikacin as per sensitivity reports of the culture isolates of Staphylococcus epidermidis and Klebsiella. The ulcers were well defined, punched out, 0.5 to 3 cm in diameter with erythematous margins and seropurulent discharge. A punch biopsy revealing neutrophilic infiltrate was described as ‘non-specific’. Repeated use of Illuminate for many days consistently failed to capture the fluorescence indicative of any pathogenic organisms, although repeated pus swabs showed growth of coagulase-negative Staphylococcus [Figure 1b]. Deep excisional biopsy thence demonstrated a dense band of neutrophilic infiltrate and perivascular lymphocytes suggestive of pyoderma gangrenosum. The patient ultimately responded well to corticosteroids (systemic and intralesional) without any antibiotic coverage.

- Clinical image of recalcitrant thigh ulcers

- Lack of fluorescence on imaging with “Illuminate”
Case 2
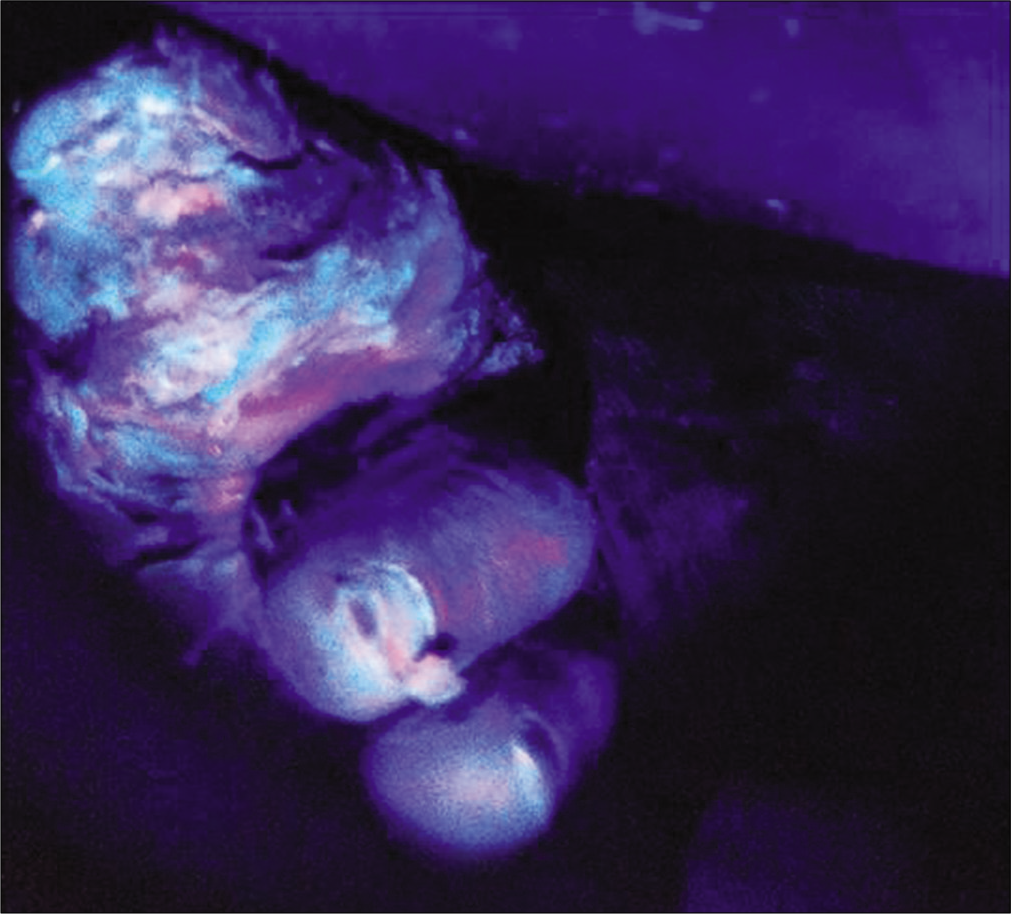
A 58-year-old female, known diabetic for 10 years developed an ulcer over her left great toe [Figure 2a] along with difficulty in walking for 4 months. Escherichia coli were isolated but despite the complete course of cefoxitin as per sensitivity, the ulcer did not heal. Cyan and green fluorescence in two different areas of the ulcer examined by Illuminate indicated the co-colonization of Pseudomonas species and Escherichia coli and the same was subsequently confirmed by image-guided swabbing [Figures 2b and c]. Prompt clinical response followed with the use of ciprofloxacin.

- Ulcer on the great toe with slough
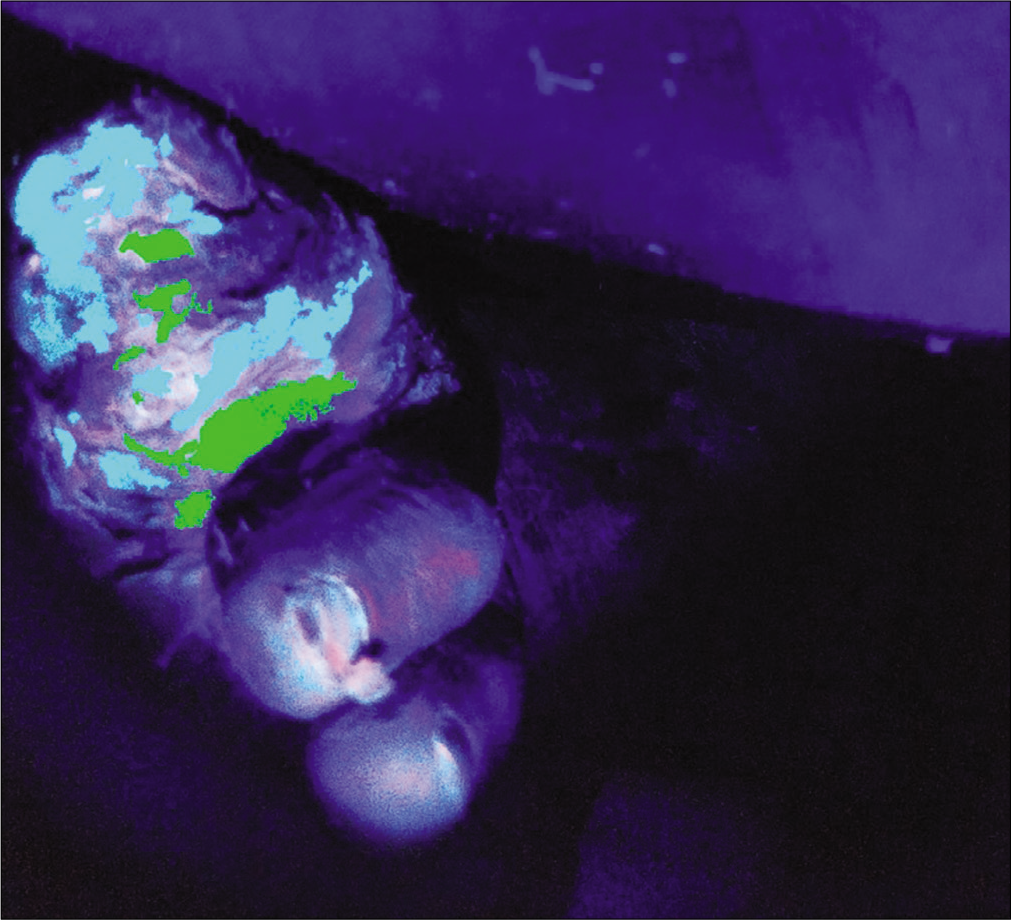
- Composite algorithmic image after processing demonstrating co-colonization
- Fluorescence highlighting areas of bacterial colonization with gram-positive species (red) and Pseudomonas
Case 3
A 45-year-old HIV-positive patient presented with a crusted ulcer on left shin for 15 days. [Figure 3a] Initial assessment with Illuminate suggested a high gram-positive bacterial load (subsequently confirmed to be methicillin-sensitive Staphylococcus aureus) and hence cotrimoxazole was given empirically. Seven days later, the sensitivity report confirmed it to be the appropriate choice, during which serial assessment demonstrated reduced fluorescence at every visit indicating a satisfactory response at the end of 10 days of treatment, obviating the need for repeated wound swabs [Figures 3b-d].
- Encrusted ulcer on the left shin
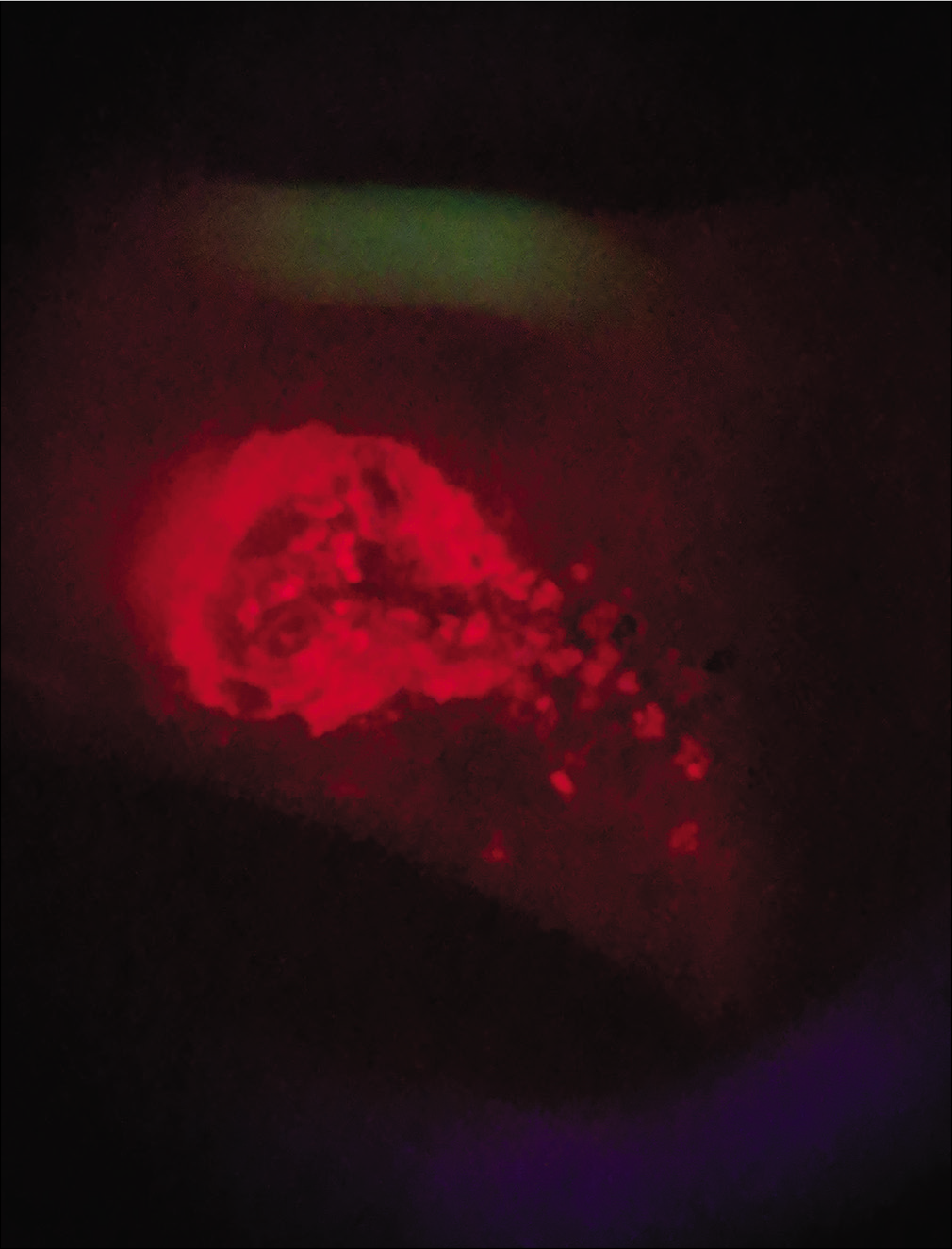
- Fluorescence at initial assessment

- Day 5 of follow-up

- Day 10 of follow-up
Case 4
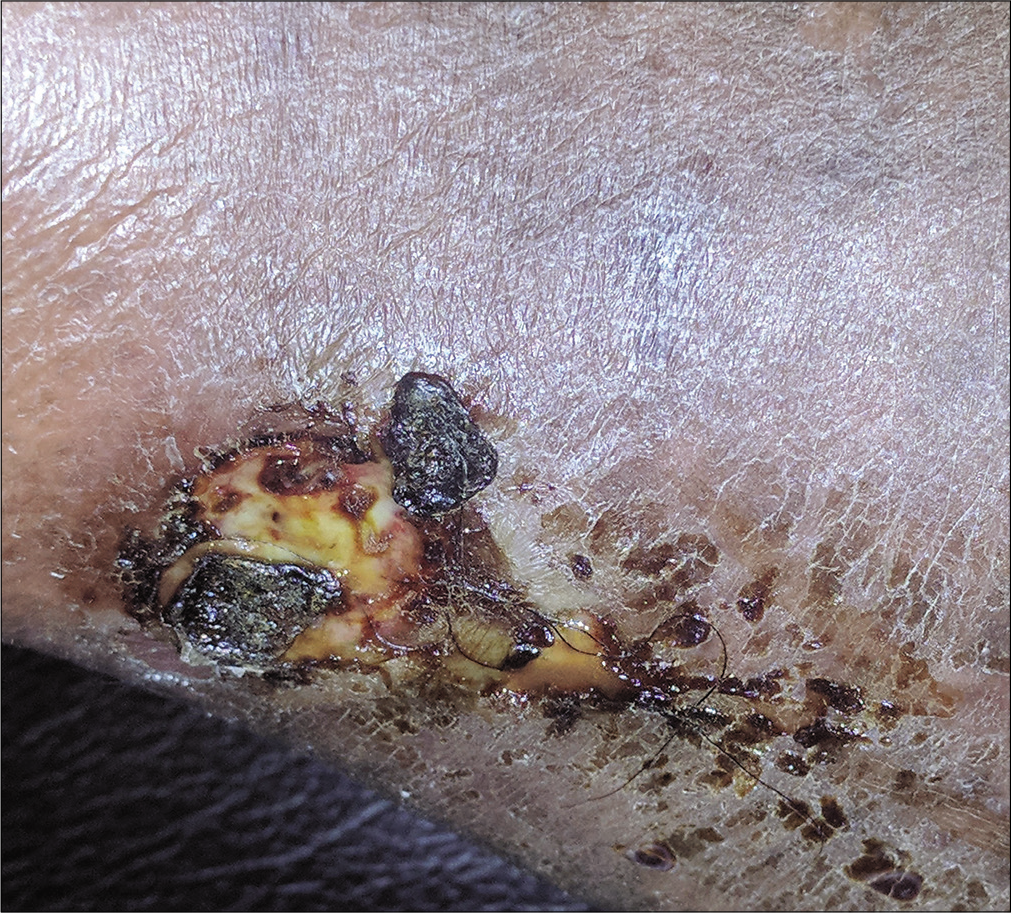
A 48-year-old male, known diabetic, presented with a malodorous, discharging ulcer over the right toe since 1 month. [Figure 4a] Earlier culture of pus swab taken from the center of the lesion yielded no growth. Clusters of methicillin-resistant Staphylococcus aureus were isolated from an image-guided swab taken from the periphery of the ulcer [Figure 4b] The wound was surgically debrided with repeated imaging till the device failed to show any fluorescence, signifying appropriate debridement depth [Figure 4c].
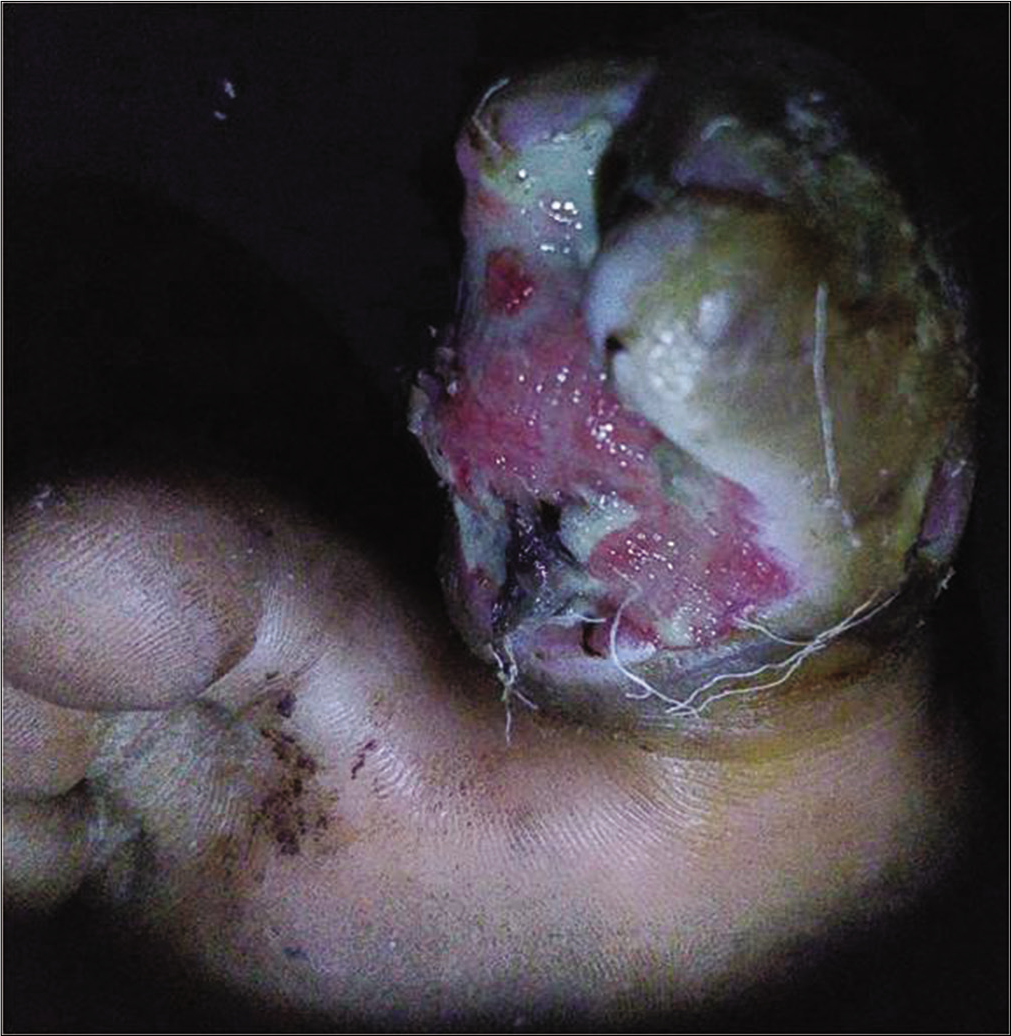
- Ulcer over the right great toe with serous discharge
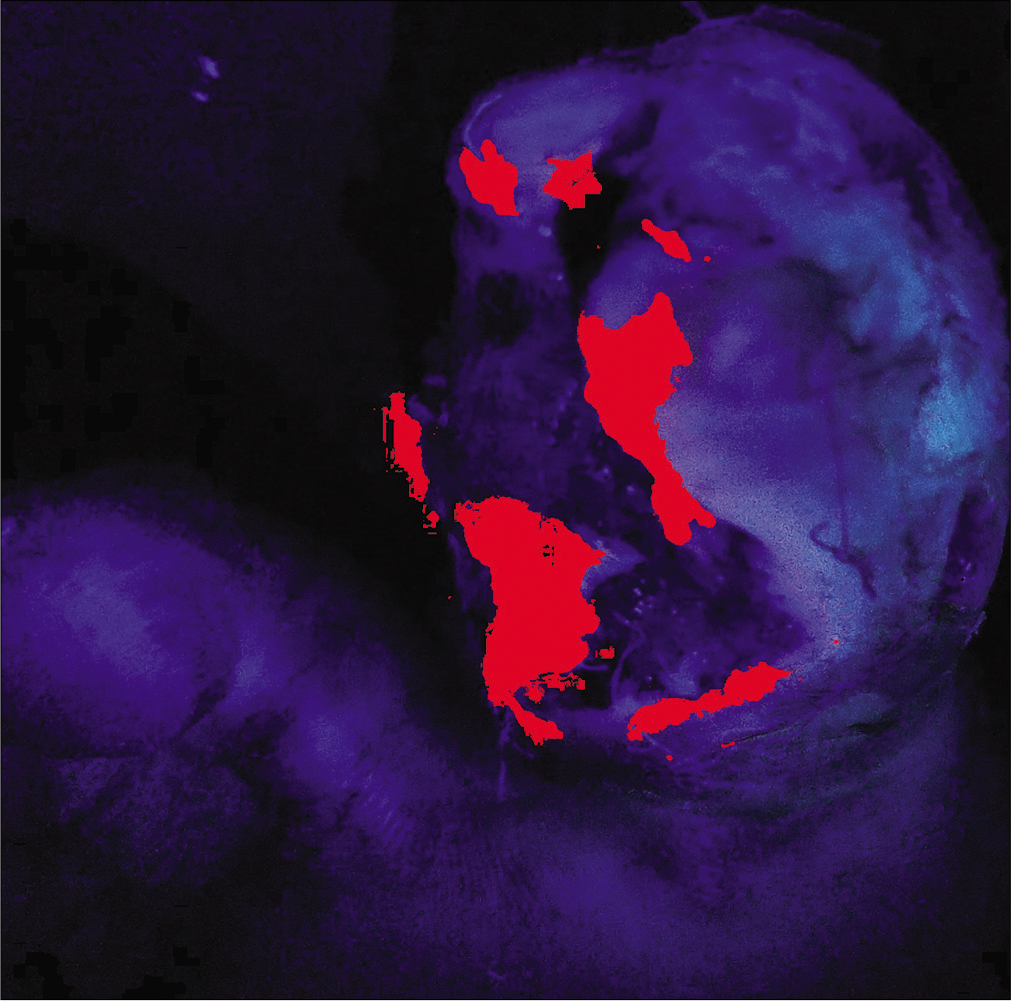
- Highlighted areas showing clusters of bacterial colonization primarily over the periphery of the wound
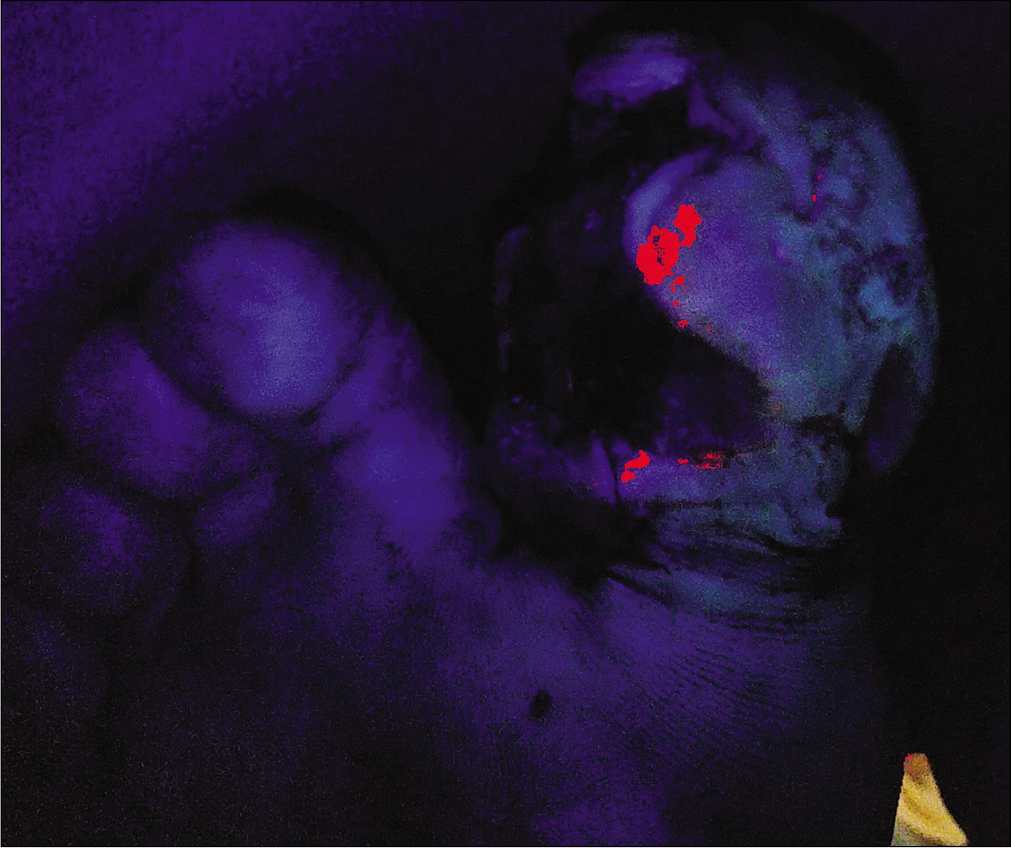
- Reduction in fluorescence evident after the initial cleaning
Discussion
Studies have shown that significant bacterial load in wounds is correctly identified only in 52.5% instances by clinical assessment as against 74.6% with fluorescent imaging-based techniques.6 Antibiotic resistance, a growing global concern can be prevented, at least in part, by antimicrobial stewardship practices.7,8 All of the key factors listed by the European Wound Management Association contributing to antimicrobial misuse in wound care, namely— diagnostic uncertainty regarding presence of bacterial infection, clinician ignorance regarding class and duration of antibiotic to be used, and patient demands for prescription of inappropriate antibiotics can be overcome by a point-of-care device providing immediate guidance.9
Our case series underscores that real-time fluorescence imaging of microorganisms can greatly facilitate the appropriate use of antibiotics. The patient with pyoderma gangrenosum, having received unnecessary and prolonged antibiotic therapy due to a misleading clinical appearance, responded promptly to systemic steroids, due to the instant result provided by this device.
Swabbing from inappropriate sites demonstrated false-negative culture results in cases 2 and 4. Sapico et al. reported only a 63% concordance of isolates between skin biopsy samples taken from the periphery and in the center of 25 pressure sores.10
The resistant-to-local-cleaning periphery of wounds was suggested to be a better swabbing site for accurate detection of isolates in some cases. Also, a mixed infection may go undetected by a single swab taken even from the area of maximal clinical involvement. Image-guided sample collection overcomes this problem.
The reduced bioburden following appropriate antibiotics/ wound debridement seen on serial imaging in cases 3 and 4 not only led to rapid healing but also supplanted the subjective clinical and/or time-consuming microbiological assessment.
Illuminate leverages the autofluorescence property exhibited by pathogens [such as nicotinamide adenine dinucleotide hydrogen (NADH), flavins] and infectious markers (pyoverdine, porphyrin) present in bacteria and fungus. NADH has a strong emission peak at 470 nm producing blue fluorescence, while flavin and porphyrin peak at around 525 nm and 620 nm corresponding to green and red fluorescence, respectively. Gram-negative organisms have a higher intensity of NADH and flavin than gram-positive organisms thus making it possible to differentiate between gram types. Pseudomonas possesses pyoverdine, a unique marker having blue-green color enabling its detection.
An image processing and machine learning algorithm processes the spectral images to detect and classify pathogens based on the intensity of autofluorescence variation amongst these biomarkers. It can detect bacterial loads as less as 103 CFU/gm (colony-forming unit per gram) allowing for early intervention (Indian patent No. 323440, 2019). Contaminants, if present, are usually at a lower density. Contamination may also occur at later stages of sample collection, processing, or in the laboratory.11
The skin itself has a natural fluorescence contributed by collagen/elastin; bacteria interact with the tissue via heme pathway and produce porphyrin displayed as red fluorescence while fungi have a characteristic blue fluorescence due to chitin and dityrosine linkages. Pseudomonas aeruginosa produces a greenish-cyan fluorescence signal due to the presence of pyoverdine. There are spectral and textural changes between the green illumination of specific organisms (especially Pseudomonas giving a cyan color) and surrounding dermis, which enable their identification. The device overlays this composite autofluorescence image on regions of infections. Gram-positive bacteria are displayed in red and gram-negative in green by comparing the difference in autofluorescence intensity.
However, limitations of the technology exist in detecting deep-seated bacterial infections and differentiating pathogenic from commensal bacteria. While an approximation of significant bacterial load is possible; the determination of accurate levels thereof still requires tissue culture. The device also cannot act as a surrogate for culture and sensitivity, which is often required to choose appropriate antibiotics in the era of drug resistance.
Point-of-care devices may substantially aid the evaluation of wounds and help institute appropriate treatment promptly by instant confirmation of infective etiology thereby reducing the investigative/financial burden, especially in resource-poor settings where facilities for microbiological evaluation may not be available.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical presentation of soft tissue infections and its management: A study of 100 cases. Niger J Surg. 2017;23:86-91.
- [CrossRef] [PubMed] [Google Scholar]
- An Indian community based epidemiological study of wounds. J Wound Care. 2004;13:323-5.
- [CrossRef] [PubMed] [Google Scholar]
- A fragment of secreted Hsp90α carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J Clin Invest. 2011;121:4348-61.
- [CrossRef] [PubMed] [Google Scholar]
- Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244-69.
- [CrossRef] [PubMed] [Google Scholar]
- When and how to culture a chronic wound: A culture is a valuable tool in wound care if used correctly. Wound Care Advisor. 2014;3:23-5.
- [Google Scholar]
- Point of care autofluorescence imaging for real time sampling and treatment guidance of bioburden in chronic wounds: First in human results. PLoS One. 2015;10:e0116623.
- [CrossRef] [PubMed] [Google Scholar]
- Tackling the threat of antimicrobial resistance: From policy to sustainable action. Philos Trans R Soc Lond B Biol Sci. 2015;370(1670):20140082.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review and meta analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial stewardship in wound care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J Antimicrob Chemother. 2016;71:3026-35.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative microbiology of pressure sores in different stages of healing. Diagn Microbiol Infect Dis. 1986;5:31-8.
- [CrossRef] [Google Scholar]






