Translate this page into:
Utility of high-frequency ultrasonography in the diagnosis of benign and malignant skin tumors
2 Department of Dermatology, Topiwala National Medical College and BYL Nair Charitable Hospital, Mumbai, Maharashtra, India
3 Department of Dermatology, MGM Medical College and General Hospital, Navi Mumbai, Maharashtra, India
4 Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Maharashtra, India
Correspondence Address:
Swagata Arvind Tambe
19/558, Udyan Society, Nehru Nagar, Kurla East, Mumbai - 400 024, Maharashtra
India
| How to cite this article: Bhatt KD, Tambe SA, Jerajani HR, Dhurat RS. Utility of high-frequency ultrasonography in the diagnosis of benign and malignant skin tumors. Indian J Dermatol Venereol Leprol 2017;83:162-182 |
Abstract
Various benign and malignant tumors may arise from the skin. These may be of epidermal, dermal, subcutaneous or appendageal origin. Skin biopsy is the gold standard for diagnosis of skin tumors. There is paucity of published data on the role of imaging modalities in diagnosis of skin tumors. High-frequency ultrasonography (7–50 MHz) is a potential non-invasive, objective modality which can be utilized in the diagnosis and localization of skin tumors. It provides valuable information about the tumor characteristics such as size, shape, depth, consistency and vascularity before invasive skin biopsy or surgery is planned. Sentinel lymph nodes in malignant melanoma can be well visualized and studied by this technique. It is also a good modality to detect local recurrence of tumors during post-operative follow up, especially those with a high likelihood of local recurrence or lesions excised with inadequate margins. High-frequency ultrasonography is additive to clinical diagnosis and can be considered a useful non-invasive method to plan the management of various skin tumors and is of prognostic value in some cases.Introduction
There is a wide spectrum of benign and malignant skin tumors originating from epidermis, dermis subcutaneous tissue and appendageal structures. Clinical acumen of the treating dermatologist is usually adequate to arrive at the provisional diagnosis. However, assessment of the depth, size and internal characteristics of these lesions are not always possible by mere clinical examination.
Inadequate depth of biopsy in a tumor may result in mis-diagnosis. Moreover, in a heterogenous tumor, complete microscopic characteristics may remain undetected with a single biopsy. Hence, some tumors may require multiple biopsies for confirmation of diagnosis.
An objective, non-invasive investigation, which may be additive to clinical examination, assessing the size, shape, characteristics and depth of skin tumors would be of great value if undertaken before biopsy. High-frequency ultrasonography is one such tool which can be used for evaluation of skin tumors before biopsy or surgery, as well as following treatment.
Ultrasound Equipment
Ultrasound equipment consists of a monitor to display an image and a lever-mounted transducer (probe). The transducer contains piezoelectric crystals. Piezoelectric crystals are made up of innumerable dipoles arranged in a geometric pattern. If a voltage is applied across the transducer in a sudden burst or pulse, the piezoelectric crystal vibrates like a cymbal and generates sound waves. As this sound pulse travels through tissues, echoes are reflected back towards the transducer; this results in re-orientation of the dipoles which in turn produces an electrical signal serving as an image display on the monitor screen.
Commonly, the real-time B-mode gray scale display is used where the depth of an echo is determined by the time taken by the sound waves to come back to the transducer. Frequency of the ultrasound used is a determining factor in penetration and resolution of the imaging system. Frequency of ultrasound is inversely related to the depth of penetration and directly to the resolution.
Skin ultrasonography can be performed by various frequencies ranging from 7.5 to 50 MHz. Resolution and depth of penetration is related to the frequency of transducers used in skin ultrasonography and are described in [Table - 1].[1] Since the structures of interest lie only a few millimeters beneath the skin surface, frequencies between 35 and 100 MHz or 1 GHz are ideal for skin ultrasonography. This is termed 'ultrasound biomicroscopy' as the resolution of the image is comparable to a low power microscopic examination. The descriptions in this article pertain to skin ultrasonography performed with 35 and 50 MHz probes using Paradigm and Sonomed ultrasonography machines (USA). To visualize deeper infiltration and vascularity of the tumors, 7.5–15 MHz multi-frequency probe with color and pulsed Doppler ultrasonography has been used (Aloka 3500 and Aloka alpha 6 ultrasonography machines, Japan) [Figure 1a] and [Figure 1b].
 |
| Figure 1a: High-frequency ultrasound machine with 7.5–15 MHz multi frequency probe with color and pulsed Doppler ultrasonography |
 |
| Figure 1b: Ultrasound biomicroscopy machine with 35 and 50 MHz probes |
Ultrasonographic Appearance of Normal Skin
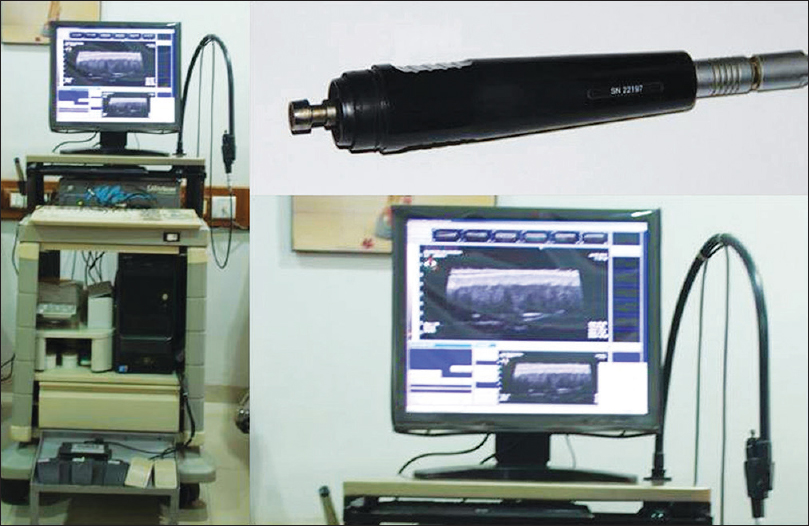
On ultrasound biomicroscopy, the epidermis is visualized as a hyperechoic epidermal entry echo. Underneath this is the homogenous isoechoic dermis within which the obliquely oriented hypoechoic hair follicles, reaching up to the subcutaneous tissue are visualized. Underneath the dermis is the hypoechoic subcutaneous tissue. The subcutaneous tissue may show linear hyperechoic structures corresponding to the septae of the fat lobules [Figure 1c].
 |
| Figure 1c: Ultrasound biomicroscopy of normal skin |
The common indications of ultrasonographic examination of skin are to measure skin thickness, study the atrophogenic effects of topical steroids and their therapeutic efficacy in chronic inflammatory and granulomatous dermatoses, localize and measure tumor thickness, to detect tumor recurrence, and study and compare therapeutic efficacy of drugs, lasers and other interventions in various skin conditions.[1]
In case of skin tumors, the three-dimensional size and outline of a tumor as well as its relationship to surrounding structures such as vessels can be evaluated. Visualization of tumor characteristics (solid, cystic, complex, calcification and necrosis) becomes easy with this tool. In addition to conventional B-mode-real time sonography, newer ultrasound techniques such as compound imaging, extended field of vision, color and pulsed Doppler sonography, elastography, three-dimensional-four-dimensional ultrasonography as well as ultrasound-guided fine needle aspiration cytology have emerged as very helpful measures in the diagnosis of skin tumors.[2]
Measurement of Tumor Thickness and Regional Lymph Nodes
High-frequency ultrasound equipment using 20–50 MHz probes are used conventionally in pre-operative assessment and post-operative follow-up of skin tumors.[3] In general, malignant neoplasms appear as hypoechoic to mixed echogenic focal lesions.[4] Using this diagnostic method, the surgeon may obtain important pre-operative information about the size (lateral extension and depth) of a cutaneous tumor. This is advantageous because the extent of the surgical procedure (margins of tumor excision and sentinel node biopsy) depends on the tumor thickness.[5] Compression with the probe should be avoided while measuring tumors as this may result in false thinning. In patients with invasive cutaneous malignancies and sentinel lymph node, high-frequency ultrasonography should be performed to detect early lymph node metastasis. Criteria for ultrasonographic characteristics of lymph node metastasis have been described.[6],[7] Some differentiating features between metastatic and reactive lymph node involvement are summarized in [Table - 2].[6],[7],[8],[9],[10],[11] If the regional lymph nodes clearly show metastatic changes on high-frequency ultrasonography, the sentinel lymph node biopsy can be omitted; in this situation, a total lymph node dissection of the region should be performed after exclusion of additional systemic metastases by further staging procedures such as magnetic resonance imaging with contrast and positron emission tomography scan. This would improve the scope of tumor management by radiotherapy, cryotherapy or surgery.

High-Resolution Ultrasonography of Benign Skin Tumors
Compound nevus
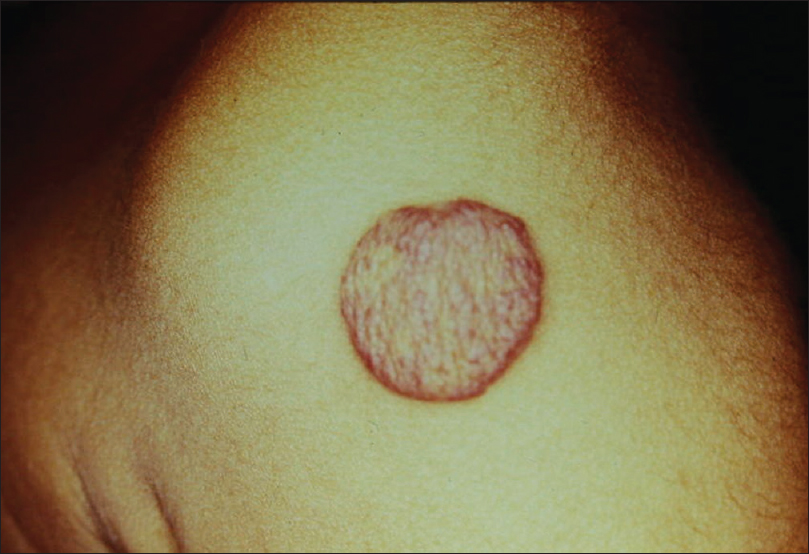
Compound nevus is a common benign growth on skin. The diagnosis is clinical but sometimes atypical presentations, such as large size, increased pigmentation and absence of overlying hair may pose a diagnostic difficulty; differentiation from other pigmented lesions such as seborrheic keratosis, warts or even melanoma is necessary in such cases [Figure 2a].
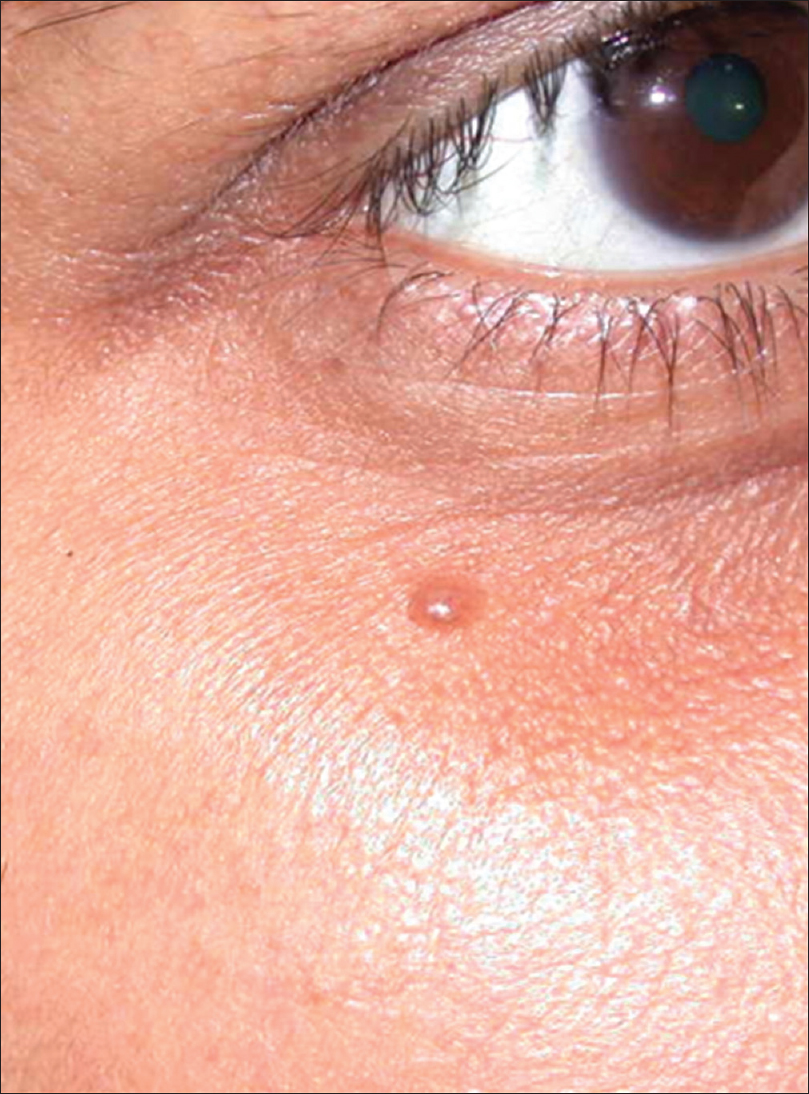 |
| Figure 2a: Compound nevus. Hyperpigmented dome-shaped papule on the right infra-orbital area |
Ultrasound biomicroscopy of compound nevus shows a well-defined hypoechoic mass lesion in the lower epidermis extending to the dermis and causing mass effect on the underlying dermis [Figure 2b]. Both epidermal and dermal involvement suggests the diagnosis of compound nevus. Seborrheic keratosis has predominantly epidermal involvement. Well-defined borders may be helpful in differentiating it from melanoma [Figure 2c].
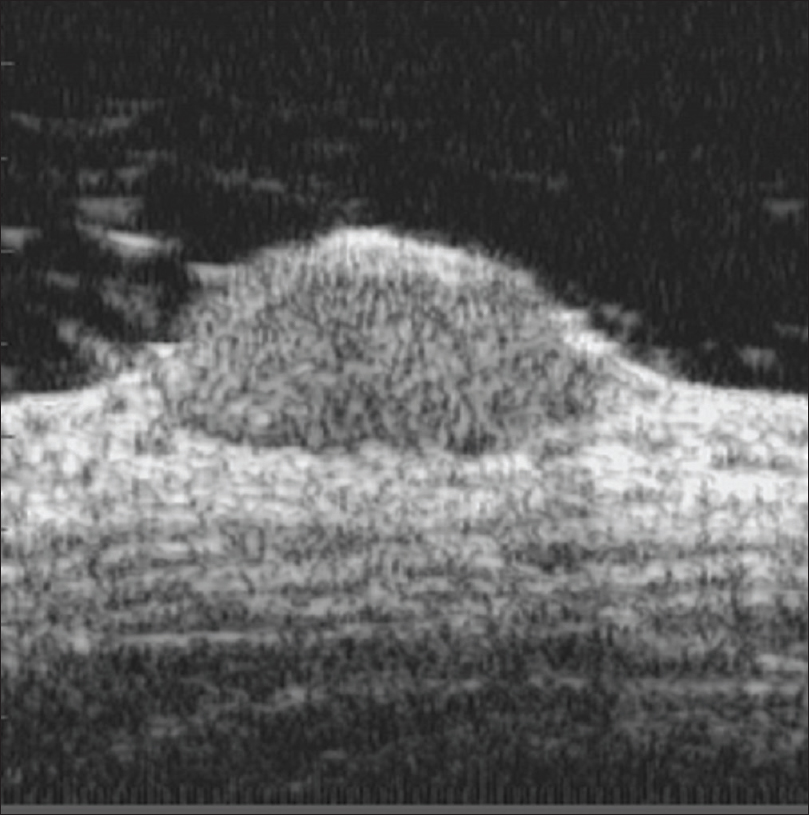 |
| Figure 2b: Compound nevus. Ultrasound biomicroscopy (50 Hz) showing a well-defined hypoechoic mass lesion in the epidermis extending into the dermis, causing mass effect on underlying dermis |
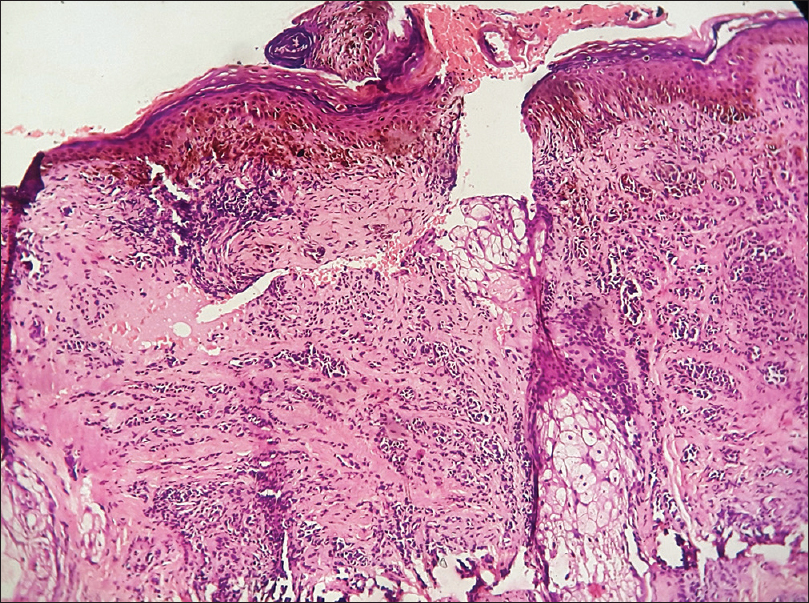 |
| Figure 2c: Compound nevus. Histopathology: Nests of nevus cells at the dermo-epidermal junction and in the dermis (H and E, ×100) |
Histopathologically, it is characterized by the presence of nests of nevus cells at the dermo-epidermal junction as well as in the dermis [Figure 2c].
Seborrheic keratosis
Seborrheic keratosis is a common epidermal tumor seen in the middle-aged and elderly. It is characterized by multiple hyperpigmented papules or plaques, often on the face [Figure 3a].
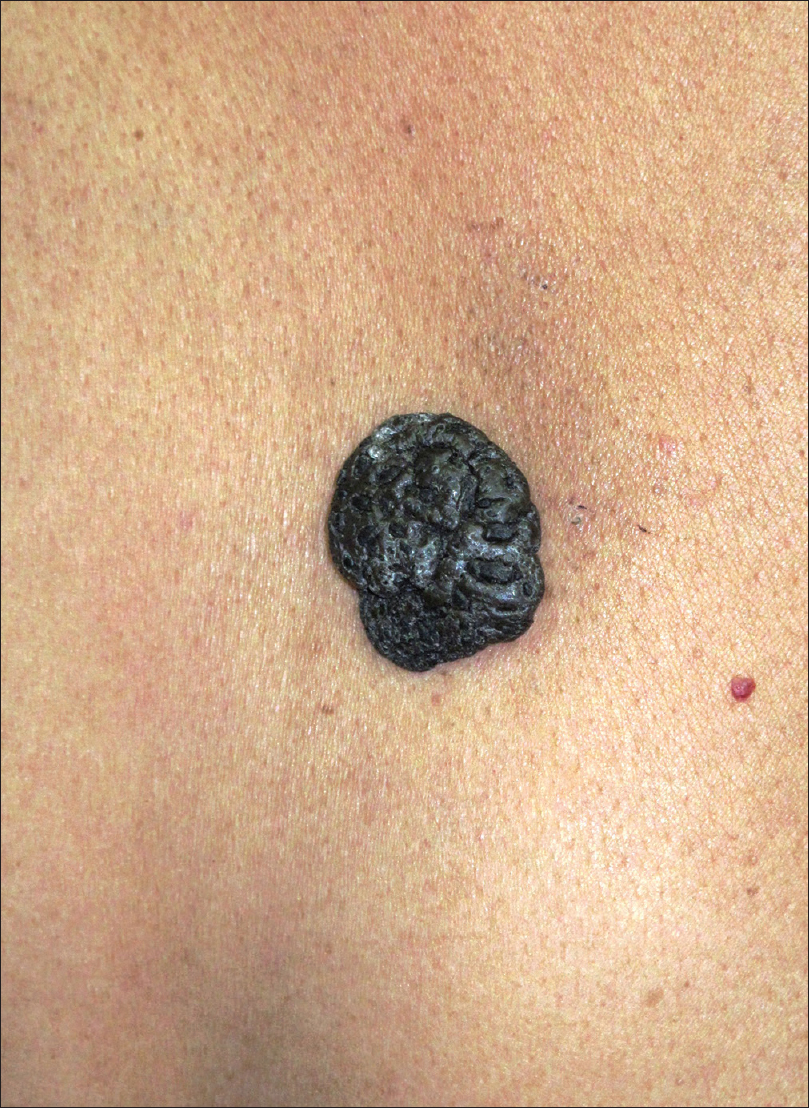 |
| Figure 3a: Seborrheic keratosis. Hyperpigmented nodule with convoluted surface located on the back |
Ultrasound biomicroscopy shows a well-defined mixed echogenic mass lesion in the epidermis with a thickened epidermal entry echo. Demarcation of normal to abnormal tissue is very sharp suggesting its benign nature [Figure 3b] and [Figure 3c].
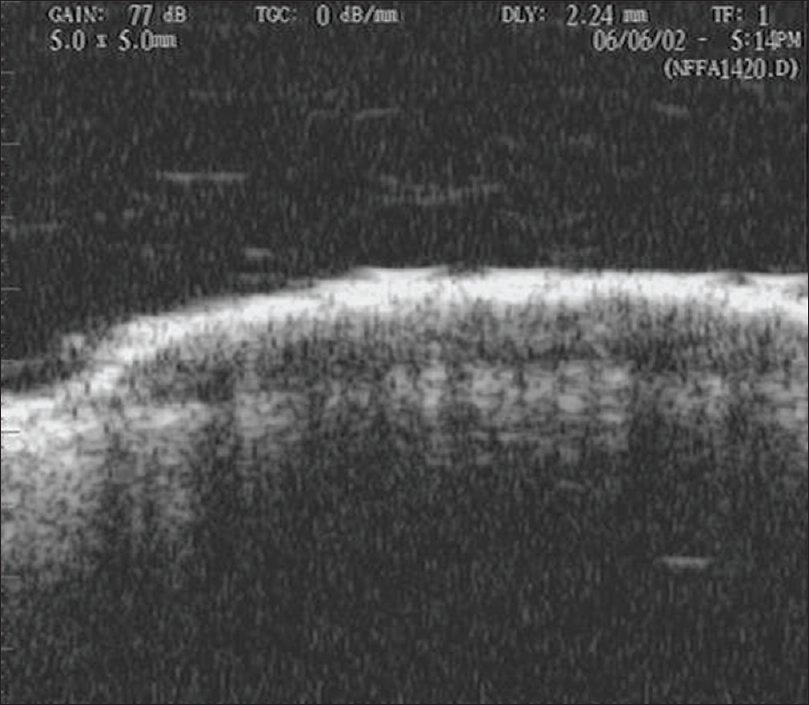 |
| Figure 3b: Seborrheic keratosis. Ultrasound biomicroscopy (50 MHz) showing a well defined mixed echogenic mass lesion in the epidermis with a thickened entry echo |
 |
| Figure 3c: Seborrheic keratosis. Demarcation of normal to abnormal tissue is very sharp |
Histopathologically, there is epidermal proliferation with hyperkeratosis, acanthosis, papillomatosis and formation of pseudohorn cysts [Figure 3d].
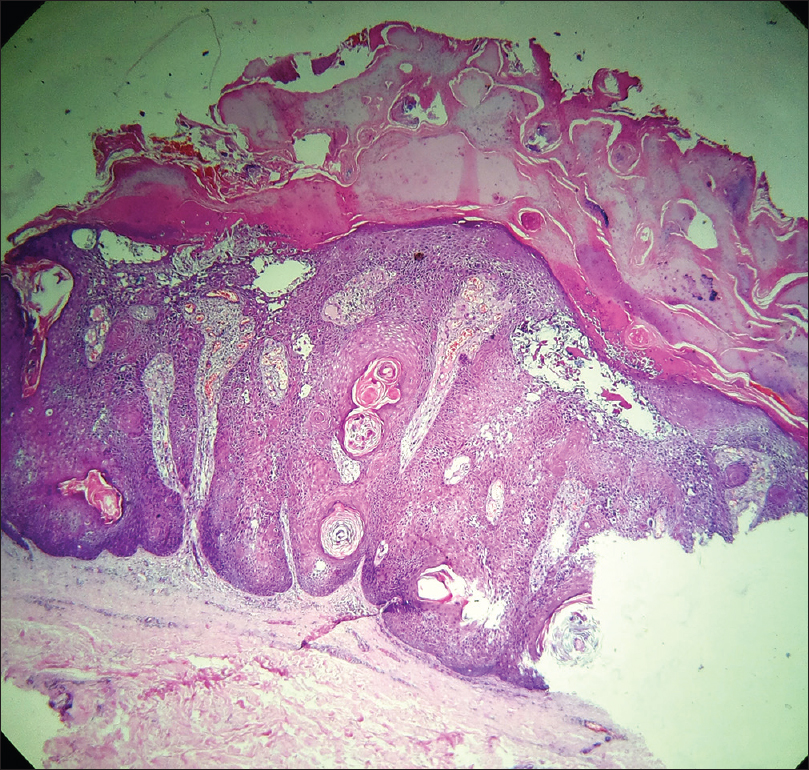 |
| Figure 3d: Seborrheic keratosis. Histopathology: Epidermal proliferation with acanthosis, papillomatosis, hyperkeratosis and psuedohorn cysts (H and E, ×40) |
Epidermoid cyst
Epidermoid or sebaceous cyst is a keratin-containing cavity lined by epidermis. It usually presents as a dermal or subcutaneous, freely mobile, nodule with a central punctum. Sometimes, the punctum may be absent [Figure 4a].
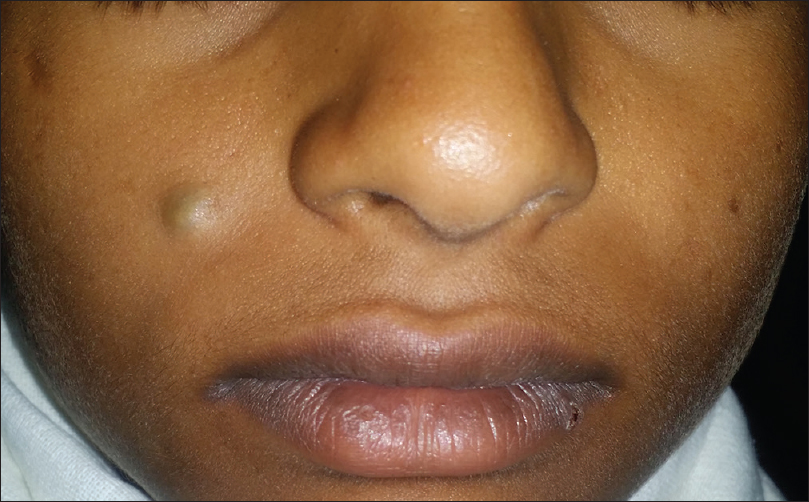 |
| Figure 4a: Sebaceous cyst. Freely mobile nodule on right cheek with central punctum |
On ultrasound biomicroscopy, there is a well-defined oval hypoechoic mass lesion in the subcutaneous plane with a punctum opening in the epidermis [Figure 4b].
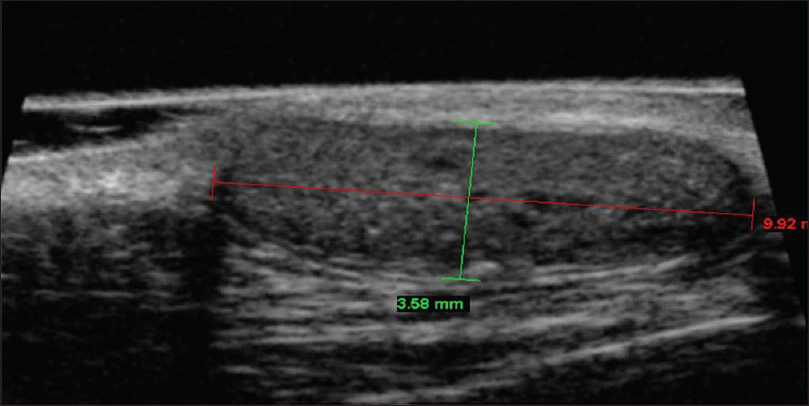 |
| Figure 4b: Sebaceous cyst. Ultrasound biomicroscopy (50 MHz) showing a well-defined, oval hypoechoic mass lesion in subcutaneous tissue with a punctum opening on to the epidermis |
Histopathologically, there is a cyst containing lamellated keratin, lined by multilayered squamous epithelium that includes the granular layer [Figure 4c].
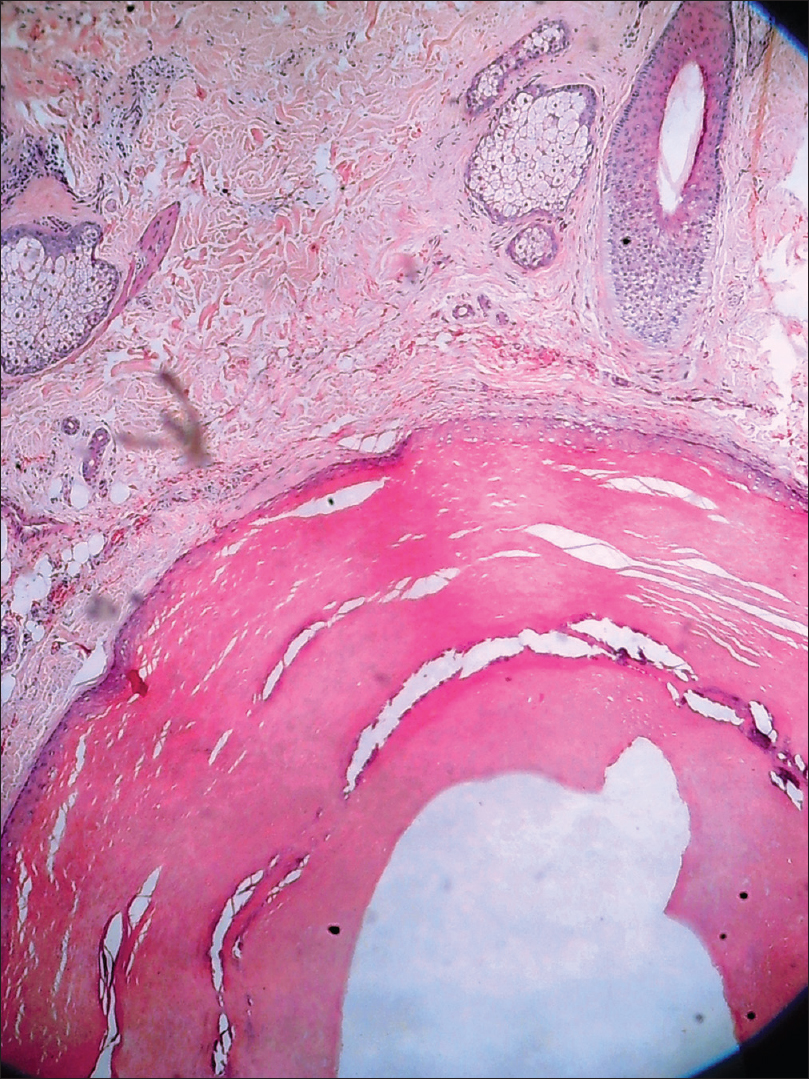 |
| Figure 4c: Sebaceous cyst. Histopathology: Cystic lesion in the dermis lined by stratified squamous epithelium filled with keratinous material (H and E, ×100) |
Pilomatricoma
Pilomatricoma is a rare benign tumor originating from hair matrix cells. It usually presents as a solitary, firm to cystic, slowly growing dermal lesion, usually over the head and neck region [Figure 5a] and [Figure 5b].
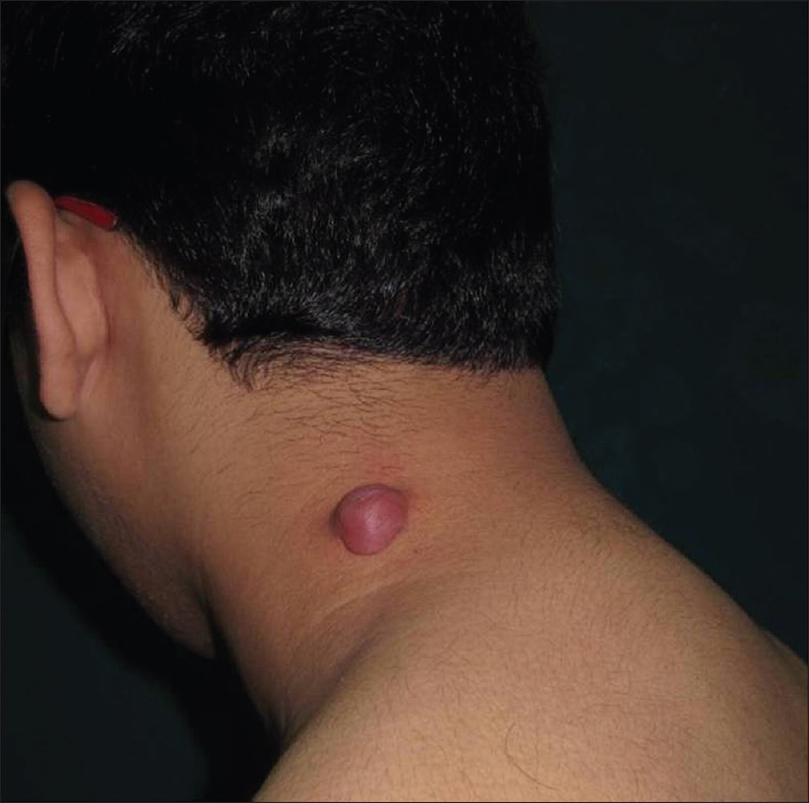 |
| Figure 5a: Pilomatricoma. Firm to cystic, solitary lesion on neck |
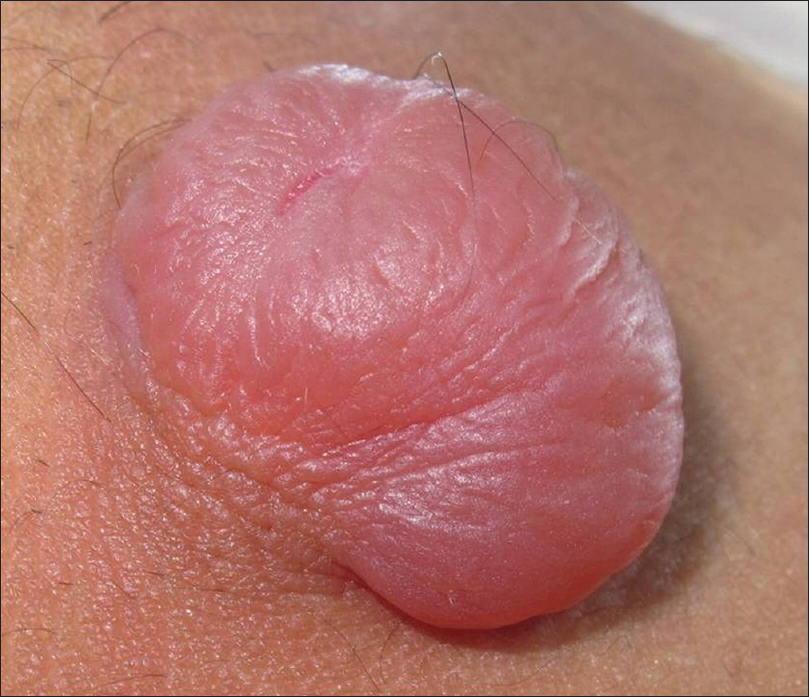 |
| Figure 5b: Pilomatricoma. The overlying skin had an erythematous hue with normal skin markings. The surface of the tumor showed multiple facets and angles |
Ultrasound biomicroscopy and high-frequency ultrasonography show a well-defined hypoechoic round mass lesion in the subcutaneous plane with multiple micro-calcifications within it [Figure 5c]. Color and pulsed Doppler study with the 15 MHz probe shows increased vascularity in the lesion with 'branching-tree' pattern of blood vessels [Figure 5d].[12]
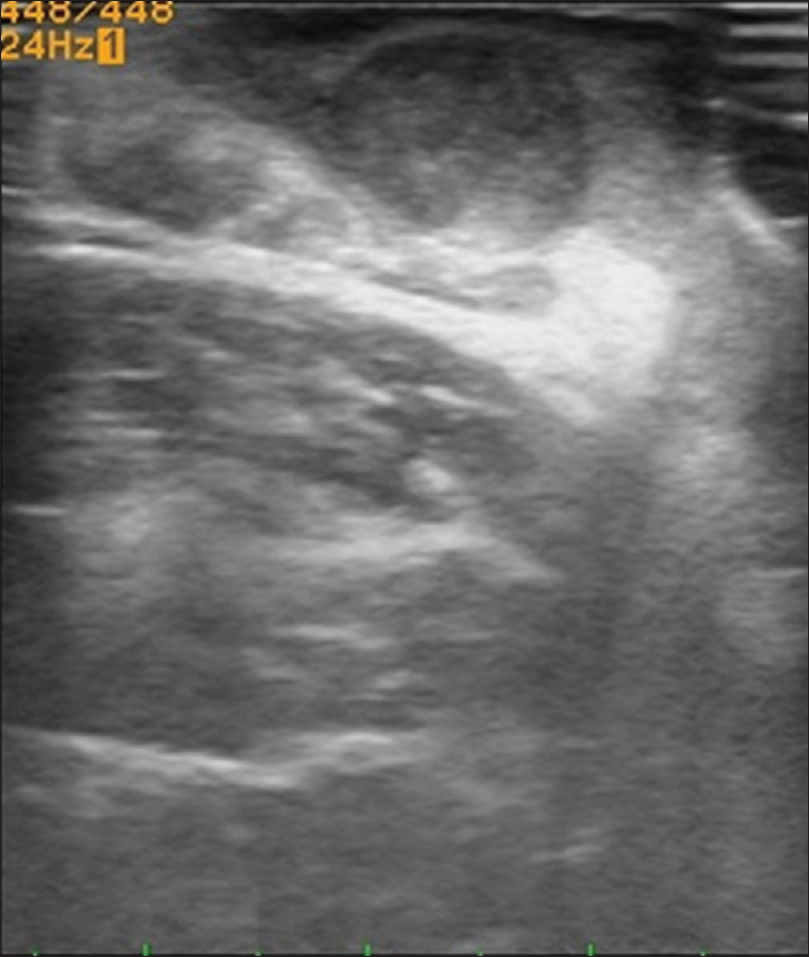 |
| Figure 5c: Pilomatricoma. High-frequency ultrasonography showing a well-defined hypoechoic round mass lesion in the subcutaneous tissue with multiple specks of calcification, causing mass effect on the underlying muscle |
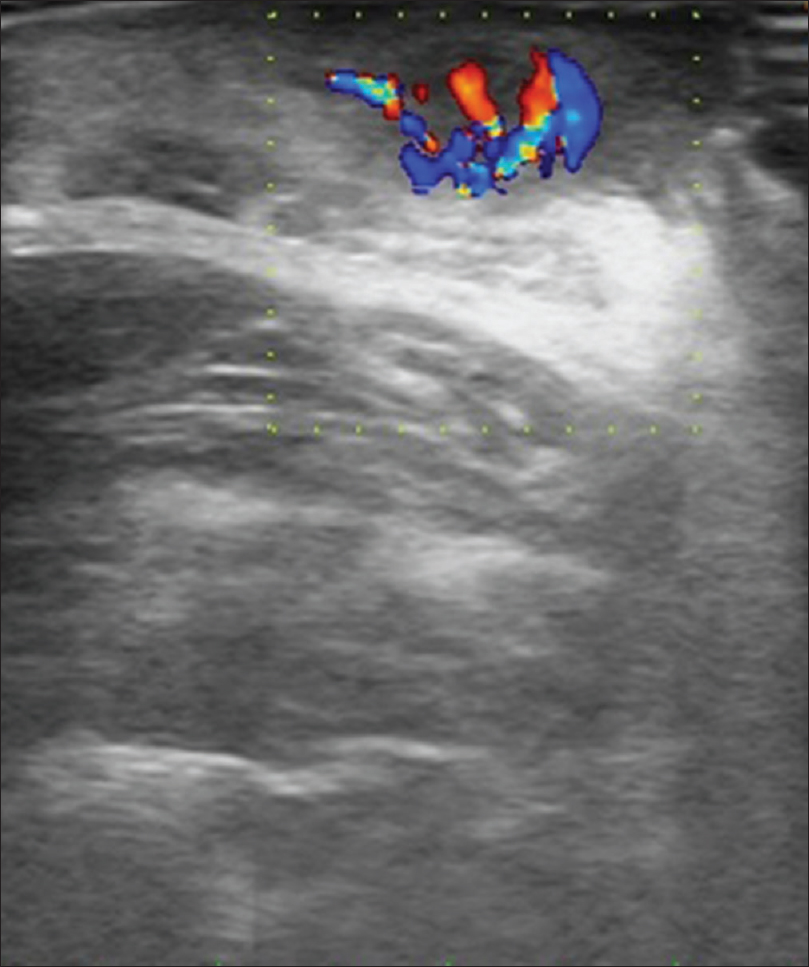 |
| Figure 5d: Pilomatricoma. Color Doppler study showing increased vascularity in the mass lesion with “branching tree pattern” |
Histopathology shows sharply demarcated deep dermal nodules surrounded by compressed fibrous tissue which may extend into the subcutaneous tissue.[Figure 5e] Islands of ghost or anucleate shadow cells are present in the center of the nodule. [Figure 5f].
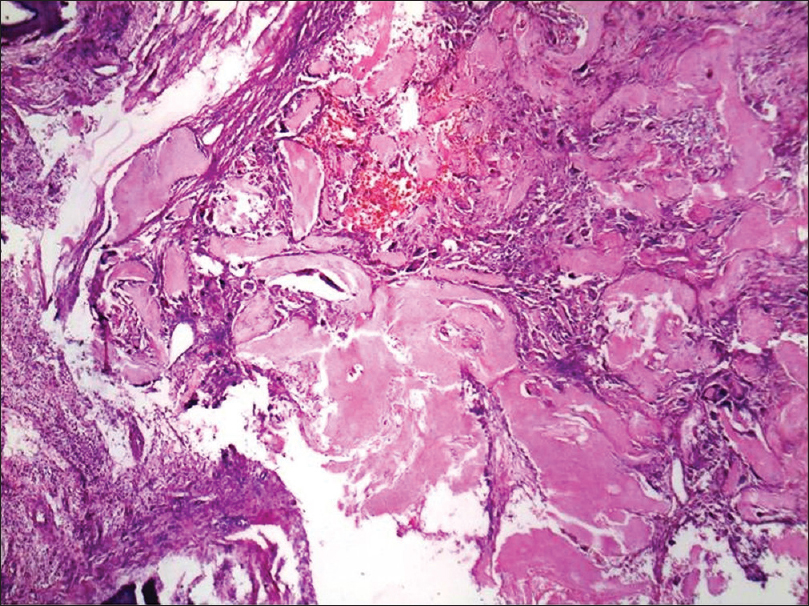 |
| Figure 5e: Pilomatricoma. Histopathology: Solid tumor mass in the reticular dermis showing basophilic nucleated cells in the periphery and anucleated cells with pink keratinized cytoplasm in the center (H and E, ×100) |
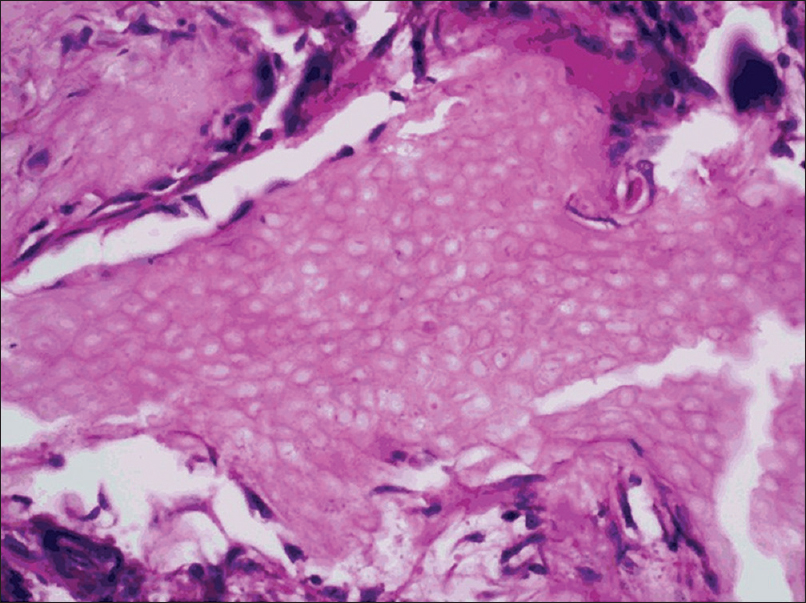 |
| Figure 5f: Pilomatricoma. Histopathology: Higher magnification showing island of pink anucleated ghost cells (H and E, ×400) |
Glomus tumor
Glomus tumor is rare, presenting as solitary or multiple, exquisitely painful, small reddish nodules on the tips of the digits or below the nail plate. Rarely, it may present as an area of localized pain without any evident lesion [Figure 6a]. In these cases, ultrasound biomicroscopy plays an important diagnostic role. It demonstrates a well-defined, hypoechoic mass lesion in the subcutaneous tissue of the proximal nail-fold, nail bed or nail apparatus. Larger lesions cause a mass effect on the nail plate, nail bed or erosion of the underlying phalanx [Figure 6b].[13]
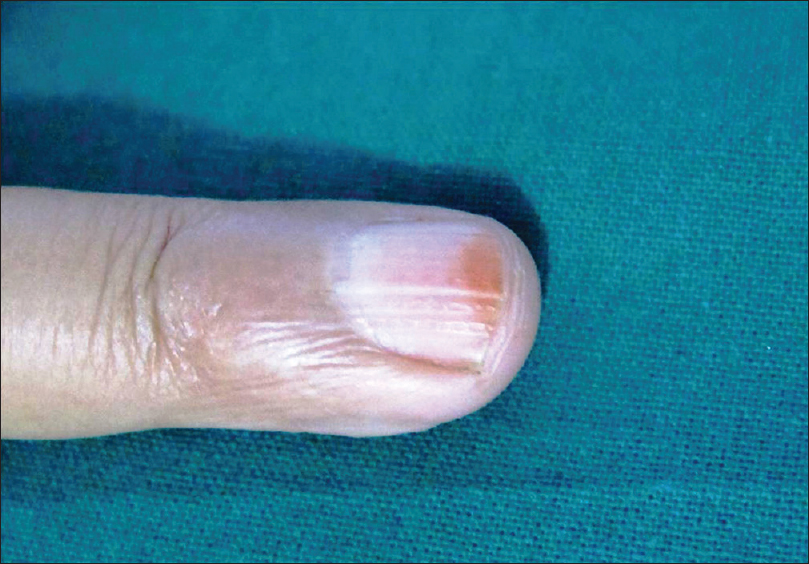 |
| Figure 6a: Glomus tumor. Minimal edema of right ring finger and longitudinal beading of the nail plate |
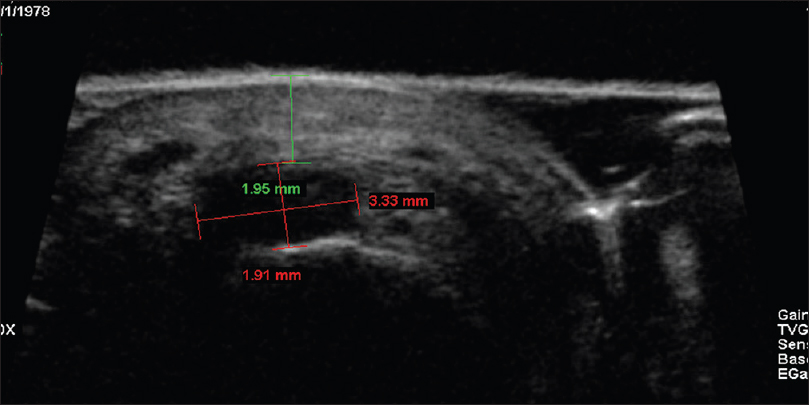 |
| Figure 6b: Glomus tumor. Ultrasound biomicroscopy in the region of maximum tenderness showing a well-defined hypoechoic mass lesion (1.91 × 3.33 mm) in the subcutaneous tissue, 1.95 mm deep to the skin surface |
Histopathology shows vascular channels surrounded by solid sheets of uniformly rounded tumor cells with eosinophilic cytoplasm and round to oval nuclei [Figure 6c] and [Figure 6d].
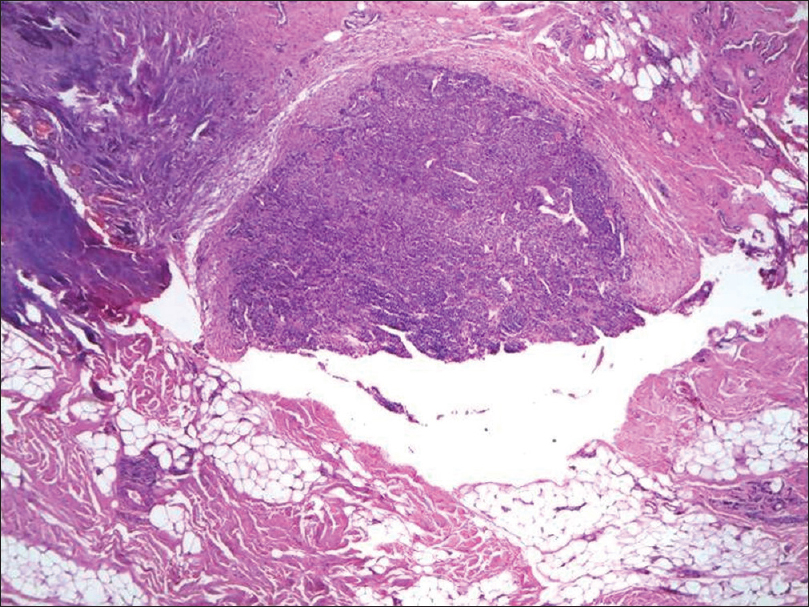 |
| Figure 6c: Glomus tumor. Excision of the tender area on histopathology showed a well-defined tumor in dermis with vascular channels (H and E stain, ×40) |
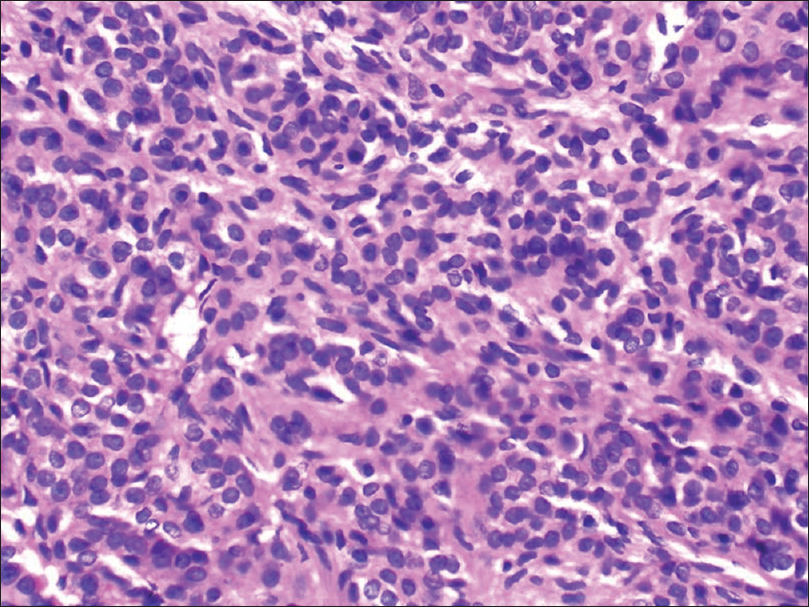 |
| Figure 6d: Glomus tumor. Histopathology: Tumor composed of vascular channels surrounded by solid sheet of uniform rounded cells with eosinophilic cytoplasm and round to oval nuclei.( H and E stain, ×400) |
Osteoma cutis
Osteoma cutis refers to the presence of bony tissue in the dermis. It can be primary (without a pre-existing lesion) or secondary (following inflammatory, traumatic and neoplastic processes). It has been described as a rare complication of severe resistant acne. It becomes a therapeutic challenge if not recognized in a timely manner.
It usually presents as solitary or multiple, small, skin-colored papules resembling closed comedones, but hard in consistency [Figure 7a] and [Figure 7b]. On removal of the contents, a hard white mass is seen [Figure 7c].
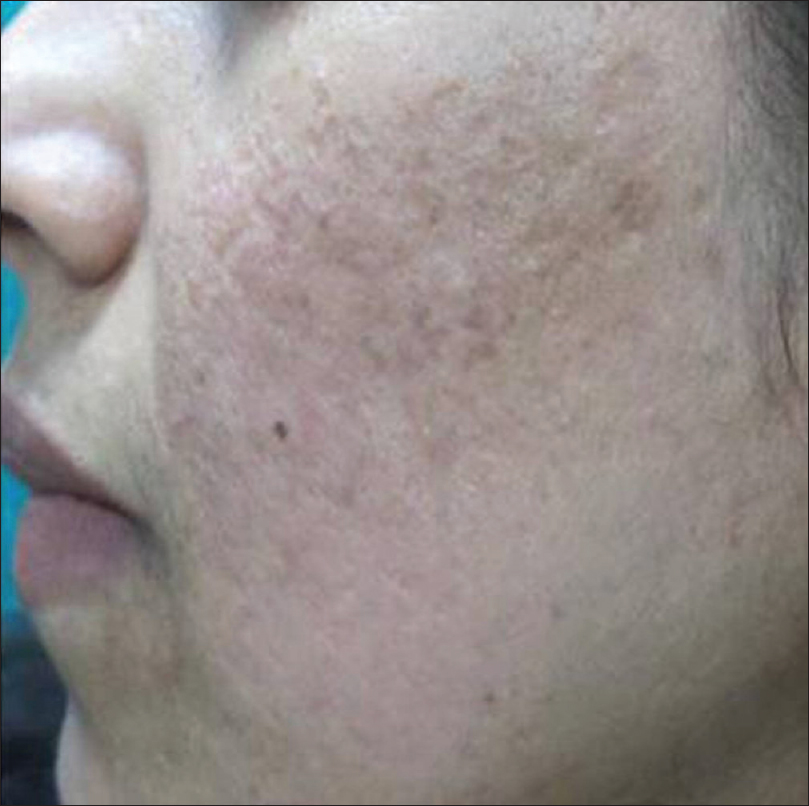 |
| Figure 7a: Osteoma cutis. Multiple small skin-colored papules resembling closed comedones, but hard in consistency |
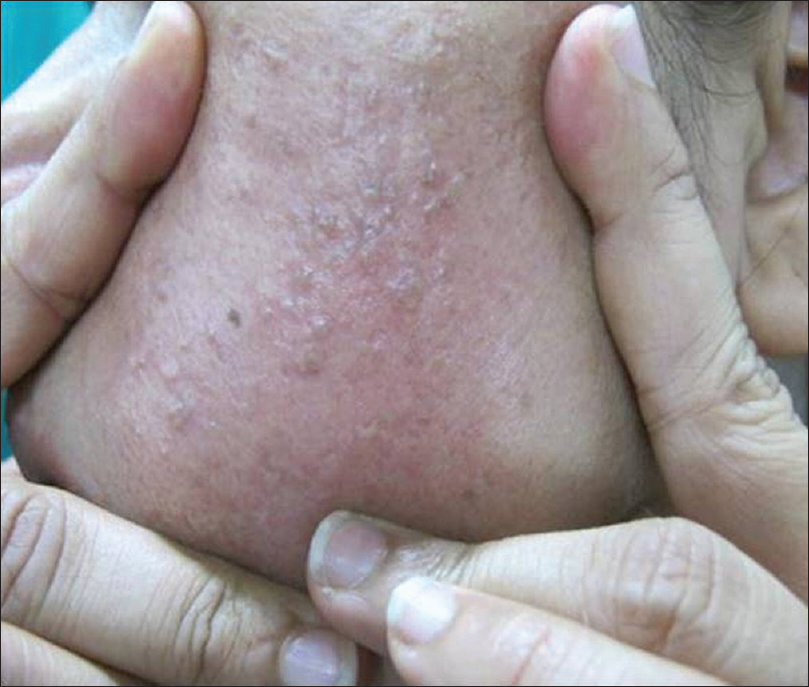 |
| Figure 7b: Osteoma cutis. Lesions becoming more apparent on stretching the skin |
 |
| Figure 7c: Osteoma cutis. White hard material expressed out after needling of the lesion |
Ultrasound biomicroscopy shows multiple curvilinear hyperechoic and calcified lesions in the upper dermis with posterior acoustic shadow [Figure 7d].
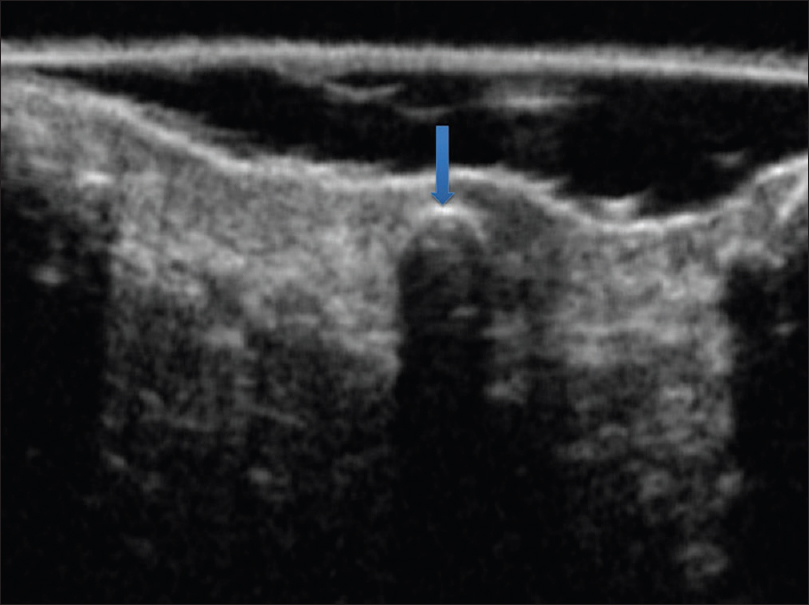 |
| Figure 7d: Osteoma cutis. Ultrasound biomicroscopy (50 MHz) showing multiple curvilinear hyperechoic lesions in the upper dermis with posterior acoustic shadowing suggestive of calcification |
Histopathology shows well-circumscribed, nodular aggregates of partially mineralized bone in the dermis [Figure 7e].
 |
| Figure 7e: Osteoma cutis. Histopathology revealed well-circumscribed, nodular aggregates of partially mineralized bone in the dermis (H and E, ×400) |
Neuroma
Neuroma is the hamartomatous proliferation of nerve sheath components. Usually, it presents as a small skin-colored, dome-shaped papule on any body part [Figure 8a].
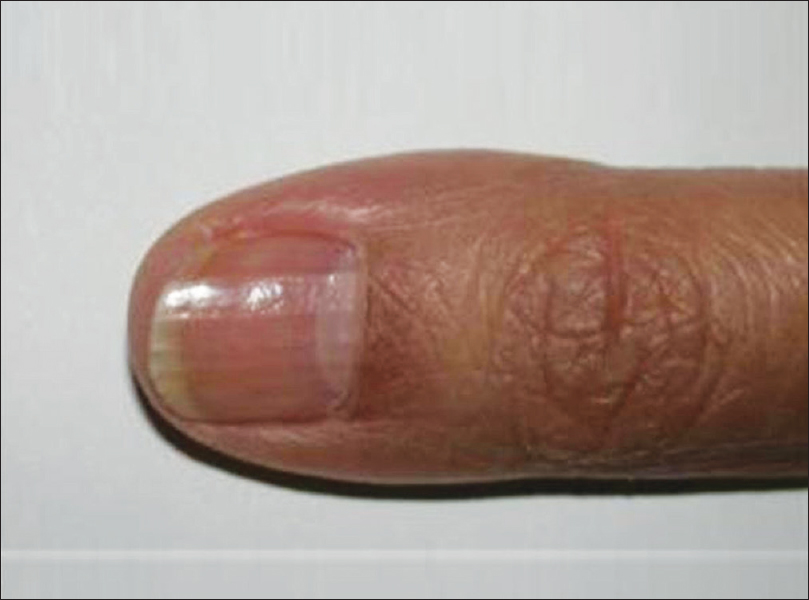 |
| Figure 8a: Neuroma. Area of tenderness just below the proximal nail-fold |
Ultrasound biomicroscopy shows a well-defined hypoechoic mass measuring 350 µm × 400 µm in the dermis extending into and causing a breach in the continuity of the overlying epidermis [Figure 8b] and [Figure 8c].[14]
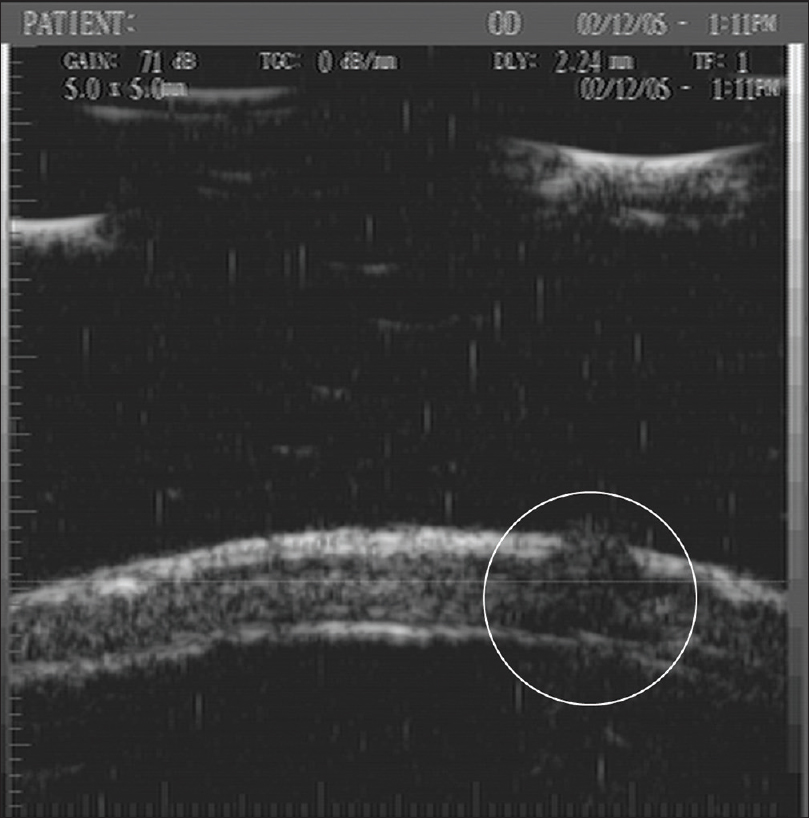 |
| Figure 8b: Neuroma. Ultrasound biomicroscopy (50 MHz) at the area of tenderness showing a well-defined hypoechoic mass (350 × 400 μm) in the dermis extending into and causing a breach in the continuity of the overlying epidermis on transverse view |
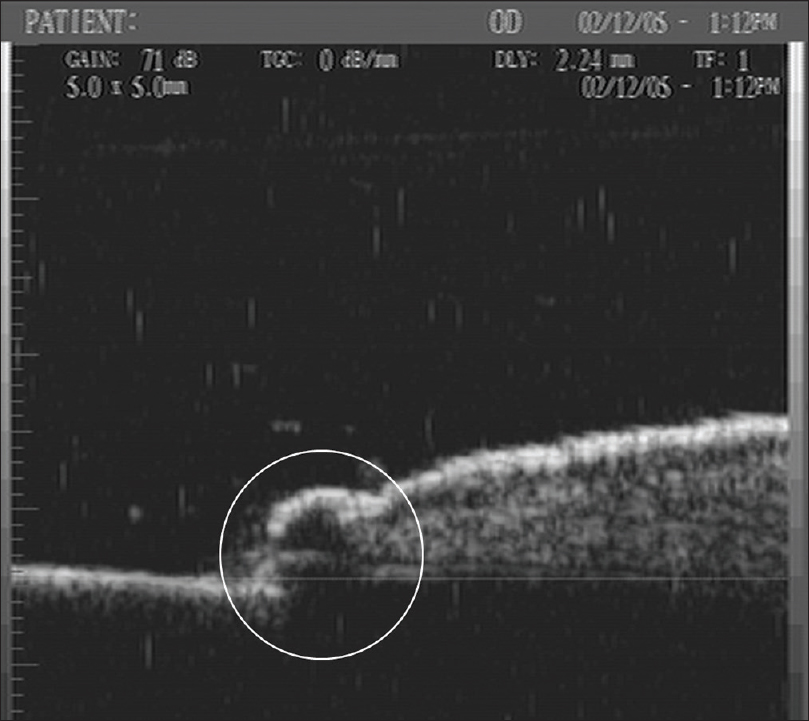 |
| Figure 8c: Neuroma. Ultrasound biomicroscopy (50 MHz) at the area of tenderness showing a well-defined hypoechoic mass lesion at proximal nail fold on saggital view |
Histopathology reveals a well-circumscribed dermal or subcutaneous nodule of varying size and shape with haphazard tangles of nerve fascicles separated by collagen. Variable amounts of chronic inflammatory infiltrate may be present [Figure 8d].
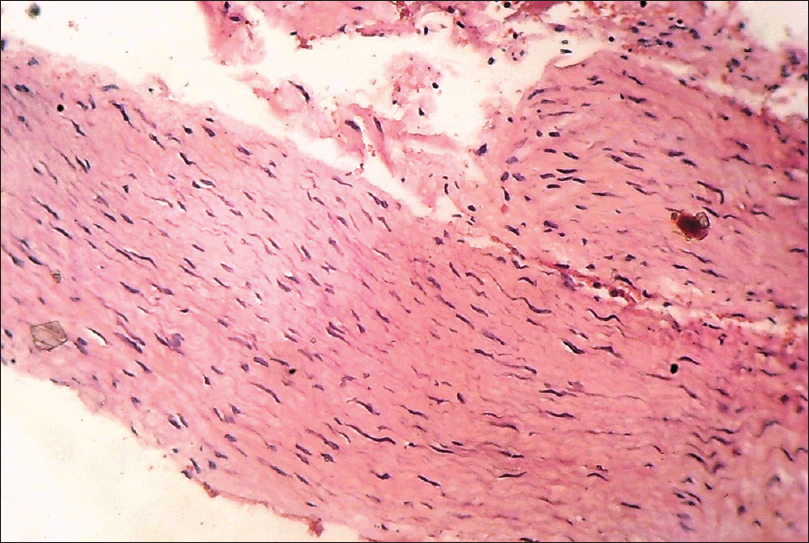 |
| Figure 8d: Neuroma. Histopathology: Well-circumscribed dermal and subcutaneous nodule with haphazard tangles of nerve fascicles separated by collagen (H and E, ×400) |
Digital mucous cyst
Digital mucous cysts are benign lesions typically located at the distal inter-phalangeal joints or in the proximal nail-fold.
The usual clinical presentation is a solitary, round-to-oval, dome-shaped, firm-to-fluctuant papule or nodule (1-10 mm). The cyst contains a viscous, gelatinous fluid that may be clear or have a yellow-tinge [Figure 9a].
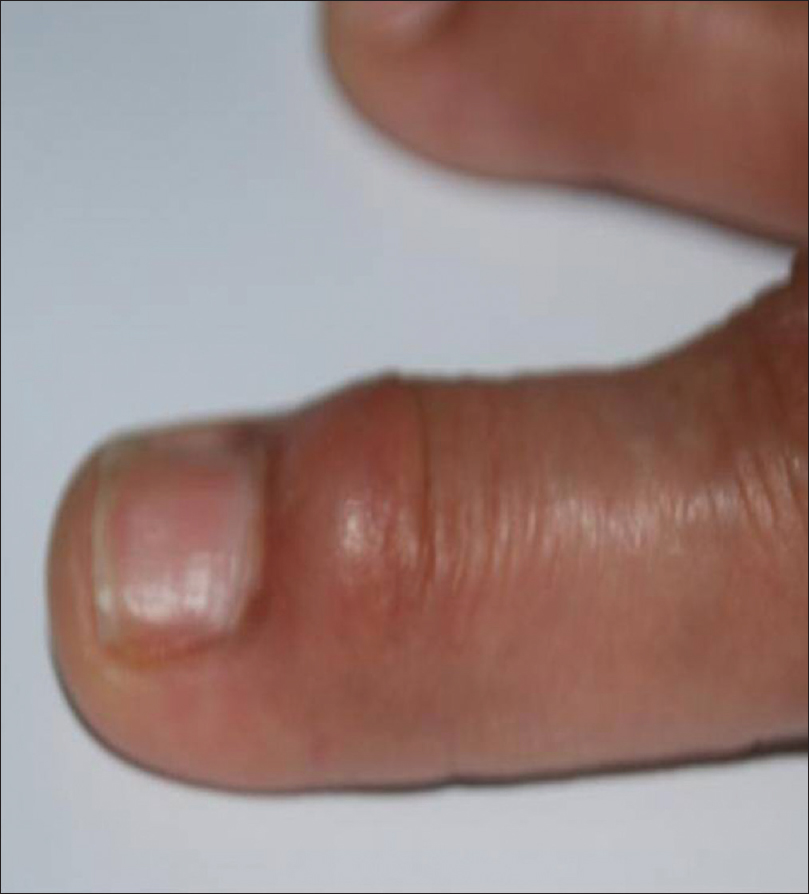 |
| Figure 9a: Digital mucous cyst. Solitary, round, dome-shaped, firm-to-fluctuant nodule in the vicinity of proximal nail-fold |
Ultrasound biomicroscopy shows a well-defined oval to round, lobulated anechoic or hypoechoic mass lesion with well-defined margins adjacent to the involved synovial compartment. A tapering margin which constitutes the “neck” of the cyst may be visualized [Figure 9b].
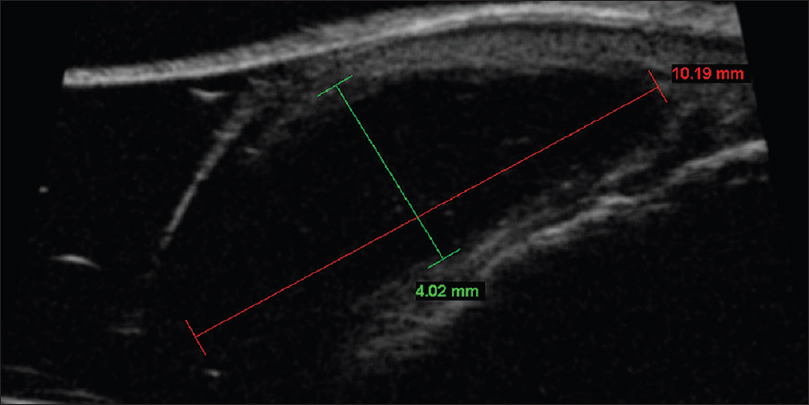 |
| Figure 9b: Digital mucous cyst. Ultrasound biomicroscopy (50 MHz): Round, lobulated mass with marked hypoechoic appearance and smooth, well-defined walls immediately adjacent to the involved synovial compartment |
Histopathology shows a pseudocyst with a fibrous capsule and myxomatous stroma with scattered fibroblasts. The surface epithelium demonstrates compact hyperkeratosis with a collarette of hyperplastic epidermis.
Lymphangioma circumscriptum
Lymphangioma circumscriptum or cutaneous lymphangioma is characterized by persistent multiple clusters of translucent vesicles containing clear lymph. Skin lesions may range from minute vesicles to small bullae, and often shows verrucous surface changes [Figure 10a].
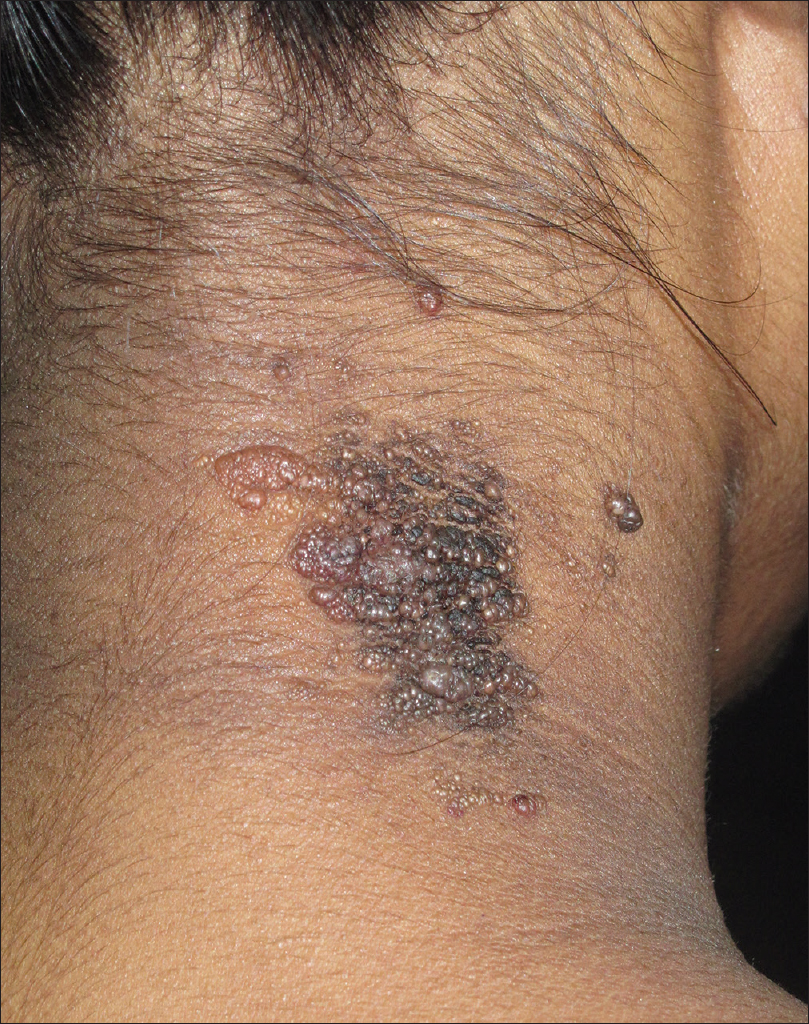 |
| Figure 10a: Lymphangioma circumscriptum. Grouped vesicular and verrucous lesions on the neck containing clear fluid |
Ultrasound biomicroscopy shows multiple, well-defined, anechoic, oval to round mass lesions in the sub-epidermal region. Absence of blood flow on color Doppler study rules out vascular malformations [Figure 10b]. High-frequency ultrasonography is done to rule out involvement of the subcutaneous tissue and underlying muscle.
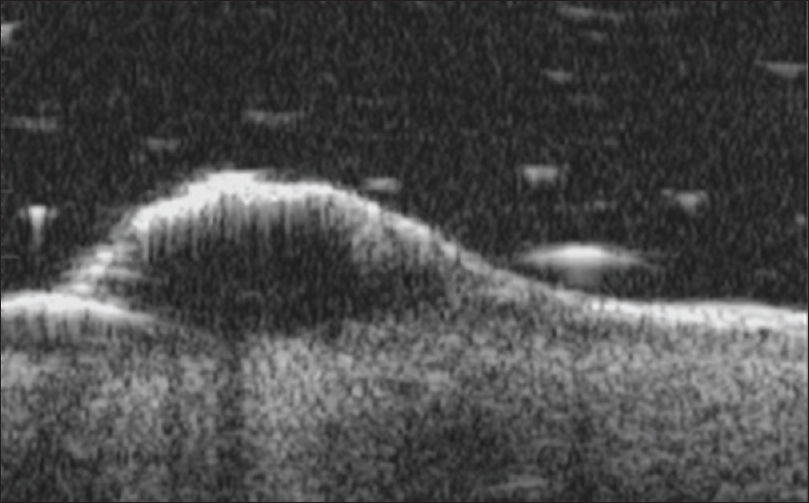 |
| Figure 10b: Lymphangioma circumscriptum. Ultrasound biomicroscopy (50 MHz) showed a well-defined anechoic oval to round mass lesions noted in the sub-epidermal region |
Histopathology reveals dilated lymphatic channels in the papillary dermis. Overlying epidermis may show hyperkeratosis and acanthosis. These channels are numerous in the upper dermis and often extend to the subcutis. The lumen is filled with lymph and the channels are lined by flat endothelial cells [Figure 10c].
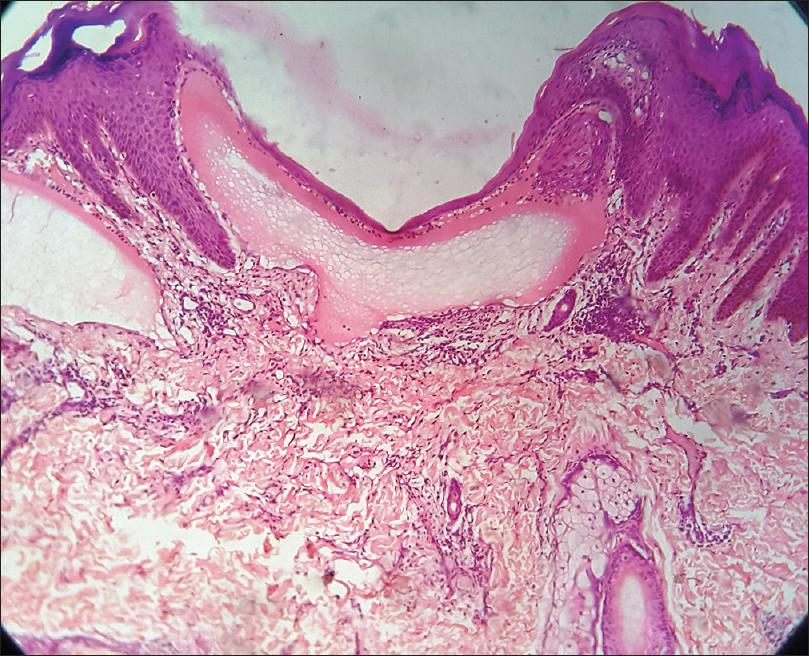 |
| Figure 10c: Lymphangioma circumscriptum. Histopathology: Hyperkeratosis and acanthosis with dilated lymphatic channels in the papillary dermis. The channels are lined by flat endothelial cells and the lumen is filled with lymph (H and E, ×40) |
Hemangioma
Infantile hemangiomas are benign vascular neoplasms with a characteristic clinical course characterized by early proliferation followed by spontaneous involution. In the early stage, it may present as an erythematous macule or patch. In the proliferative phase, lesions have a dome-like, lobulated or plaque-like morphology [Figure 11a].
 |
| Figure 11a: Hemangioma. Erythematous lobulated plaque on left arm |
On ultrasound biomicroscopy, a well-defined nodular hypoechoic mass lesion is seen in the epidermis and the subepidermal region [Figure 11b]. Color Doppler study may show vascularity in the mass lesion.
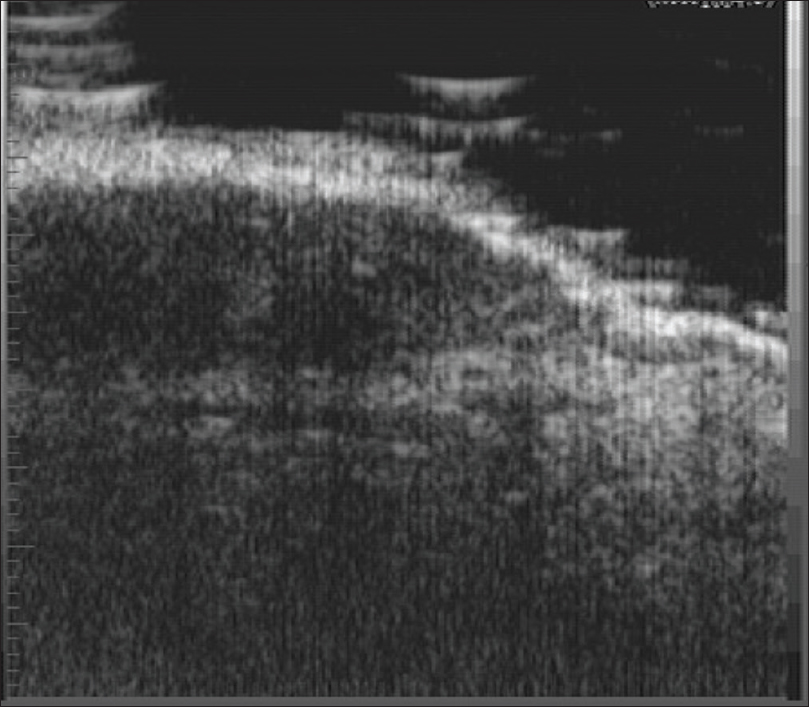 |
| Figure 11b: Hemangioma. Ultrasound biomicroscopy (50 MHz) showing a well-defined nodular hypoechoic mass lesion in the epidermis extending to the subepidermal region |
Biopsy is usually not done as the diagnosis is clinical. Histopathology shows non-encapsulated aggregates of closely packed, thin-walled capillaries usually with an endothelial lining. Blood-filled vessels are separated by scanty connective tissue [Figure 11c].
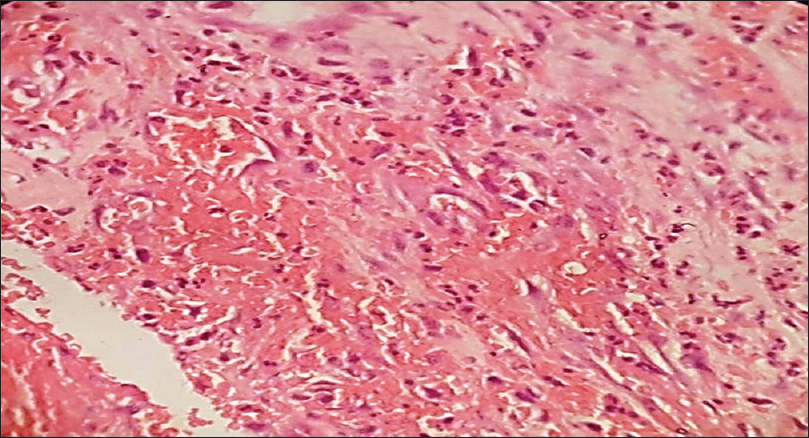 |
| Figure 11c: Hemangioma. Histopathology: Tumor composed of aggregates of closely packed, thin-walled capillaries with endothelial lining separated by scanty connective tissue (H and E, ×400) |
Arteriovenous malformations
Arteriovenous malformations are fast-flow vascular lesions composed of directly connected, dysmorphic arterial and venous channels without an intervening capillary bed.
When fully developed, the color of arteriovenous malformations deepens, with increased erythema or a bluish hue, raised local temperature, a palpable thrill and bruit [Figure 12a].
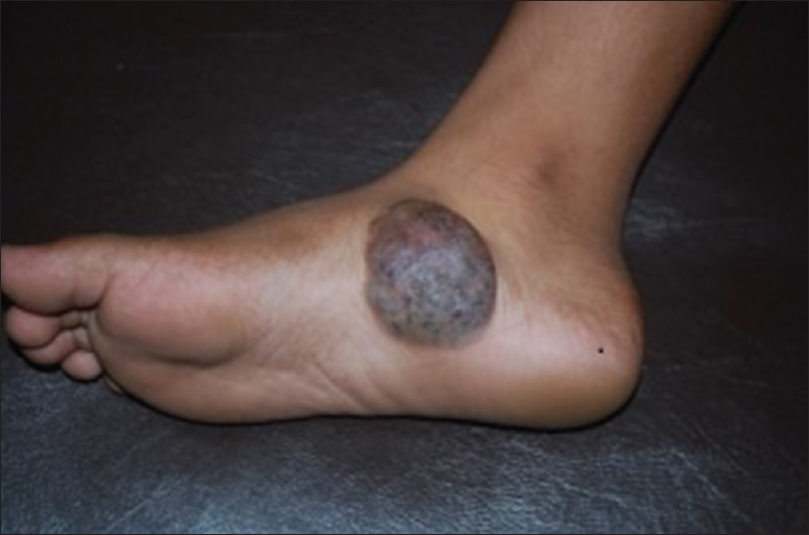 |
| Figure 12a: Arteriovenous malformations. Bluish compressible nodule on the medial aspect of right foot with raised local temperature, palpable thrill and bruit |
High-frequency ultrasonography shows a well-defined non-homogeneous mass lesion in the dermis extending to the subcutaneous tissue [Figure 12b]. On color and pulsed Doppler study, arteriovenous blood flow is noted in the mass lesion [Figure 12c].
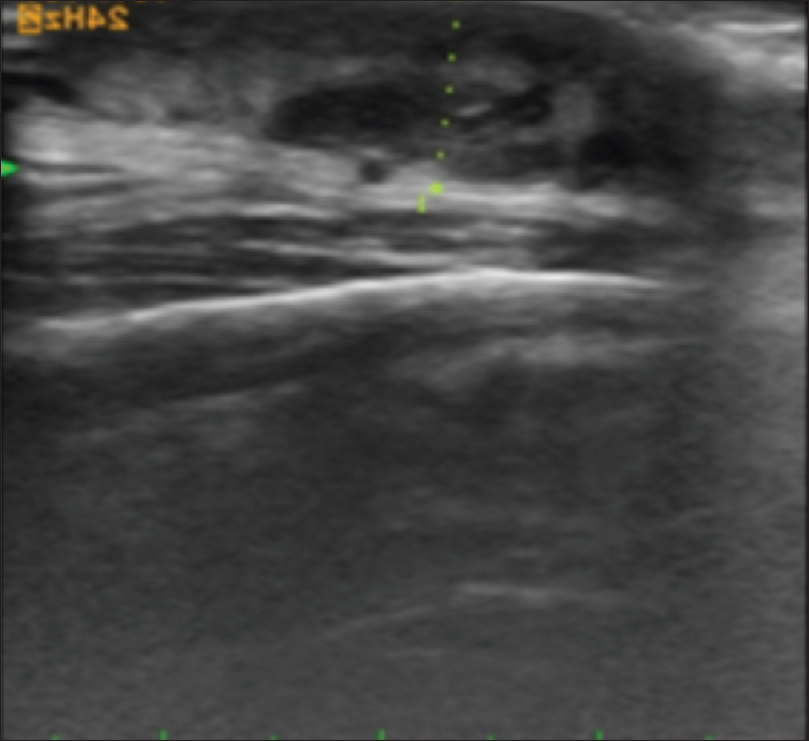 |
| Figure 12b: Arteriovenous malformations. High-frequency ultrasonography showing a well-defined non-homogeneous mass lesion in the dermis and the subcutaneous tissue |
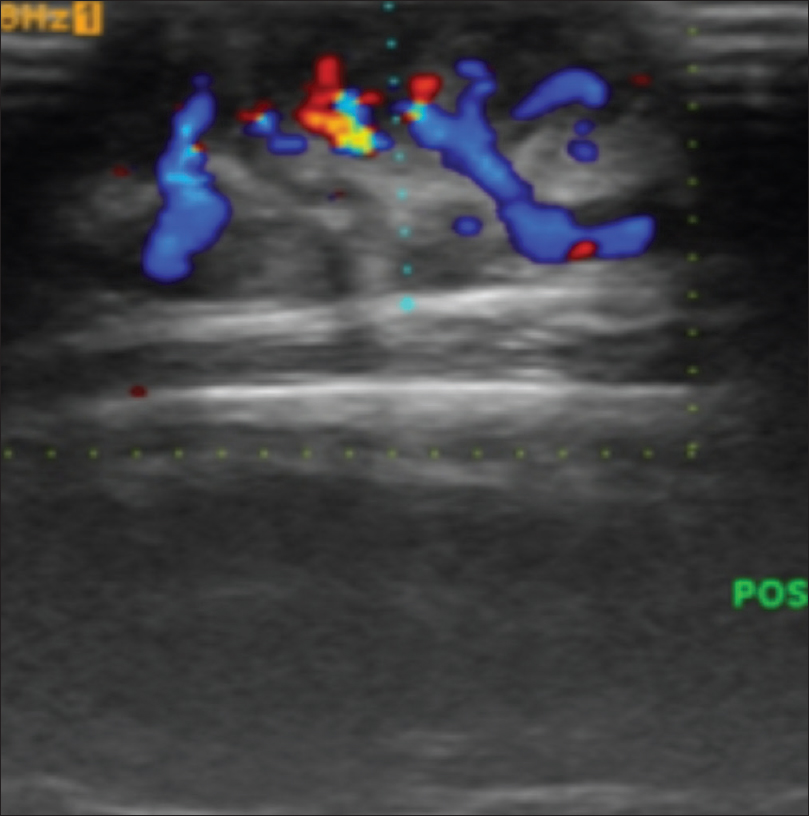 |
| Figure 12c: Arteriovenous malformations. Color Doppler and power Doppler study showing arterio-venous blood flow is noted in the mass lesion |
Diagnosis is confirmed by clinical examination and color Doppler study.
High-Resolution Sonography of Malignant Skin Tumors
Bowen's disease
Bowen's disease is a neoplastic skin lesion, also known as squamous cell carcinoma in situ. It usually presents as a solitary, sharply demarcated, scaly plaque with irregular borders. However, the morphology may be variable from erythematous, scaly patches to hyperkeratotic, crusted, fissured, or ulcerated lesions [Figure 13a].
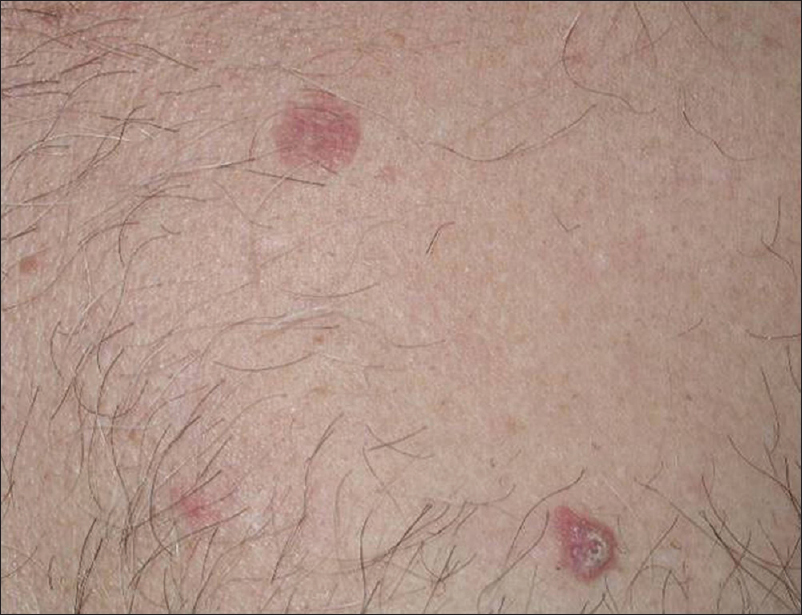 |
| Figure 13a: Bowen's disease. Multiple erythematous scaly plaques |
Ultrasound biomicroscopy examination (50 MHz) shows a nodular mass lesion (0.74 mm × 2.59 mm) arising from a thickened epidermal entry echo with a subepidermal hypoechoic band (0.72 mm). This feature is suggestive of epidermal location of the lesion without dermal invasion, unlike in squamous cell carcinoma [Figure 13b].
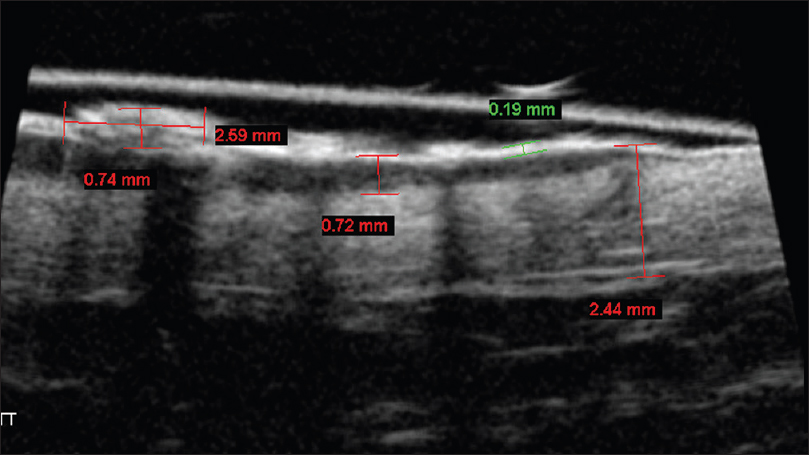 |
| Figure 13b: Bowen's disease. Ultrasound biomicroscopy (50 MHz) showing a nodular mass lesion (0.74 × 2.59 mm) arising from a thickened epidermal entry echo. A sub-epidermal hypoechoic band measuring 0.72 mm confirms that the lesion is restricted to the epidermis |
Histopathology shows full thickness epidermal hyperplasia with crowded keratinocytes that demonstrate disordered polarity, loss of maturation and nuclear pleomorphism and hyperchromasia with an intact basement membrane. [Figure 13c].
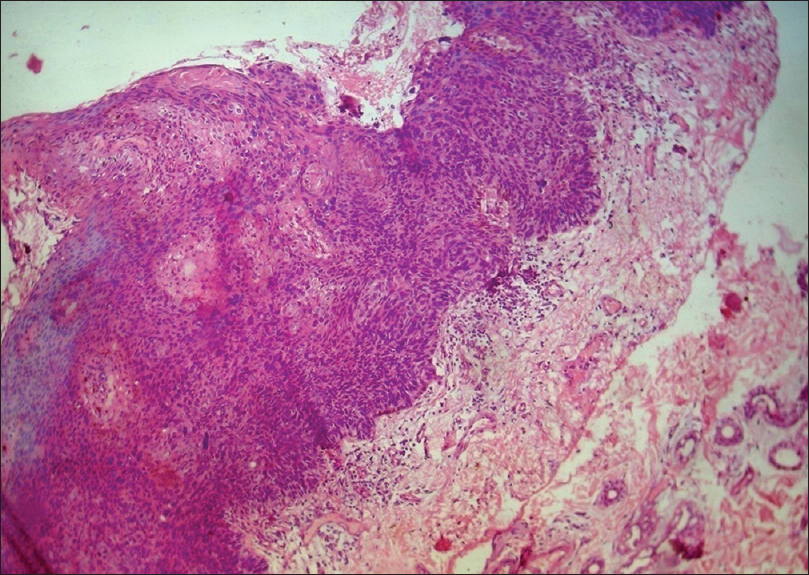 |
| Figure 13c: Bowen's disease. Histopathology: Full thickness epidermal hyperplasia with crowded keratinocytes, nuclear pleomorphism and hyperchromasia with intact basement membrane (H and E, ×100) |
Squamous cell carcinoma
Cutaneous squamous cell carcinoma is an invasive malignant neoplasm of epidermal keratinocytes showing squamous phenotypic differentiation.
The classic presentation is that of a shallow ulcer with heaped-up edges, often on sun-exposed areas. Surface changes may include scaling, crusting and ulceration [Figure 14a].
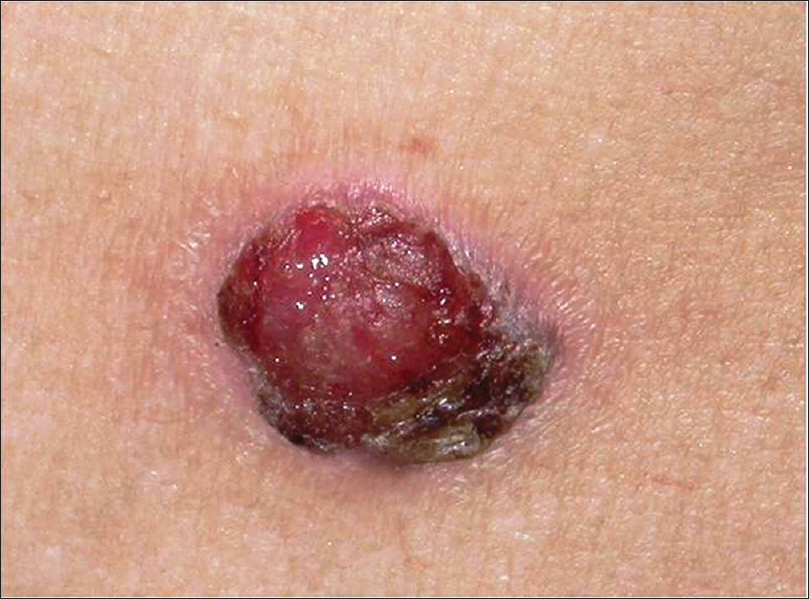 |
| Figure 14a: Squamous cell carcinoma. Solitary ulcerated plaque covered with a crust, on lateral chest wall |
Ultrasound biomicroscopy shows a well-defined nodular hypoechoic mass lesion in the epidermis, sub-epidermal region and the upper dermis. The dermal invasion differentiates it from Bowen's disease [Figure 14b].
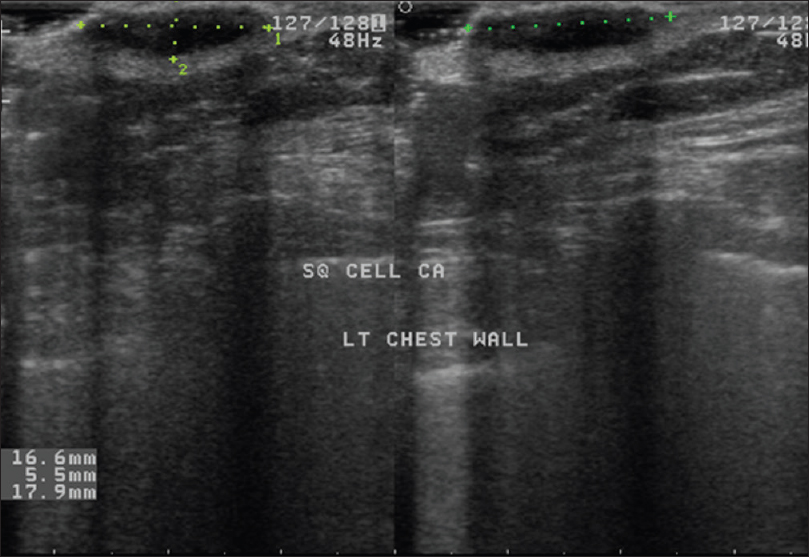 |
| Figure 14b: Squamous cell carcinoma. High-frequency ultrasonography showing a well-defined nodular hypoechoic mass lesion in the epidermis, subepidermal region and upper dermis |
Histopathology shows pseudoepitheliomatous hyperplasia, atypical squamous cells, and multiple mitotic figures. Nests of well to poorly differentiated squamous cells are present in the dermis [Figure 14c] and [Figure 14d].
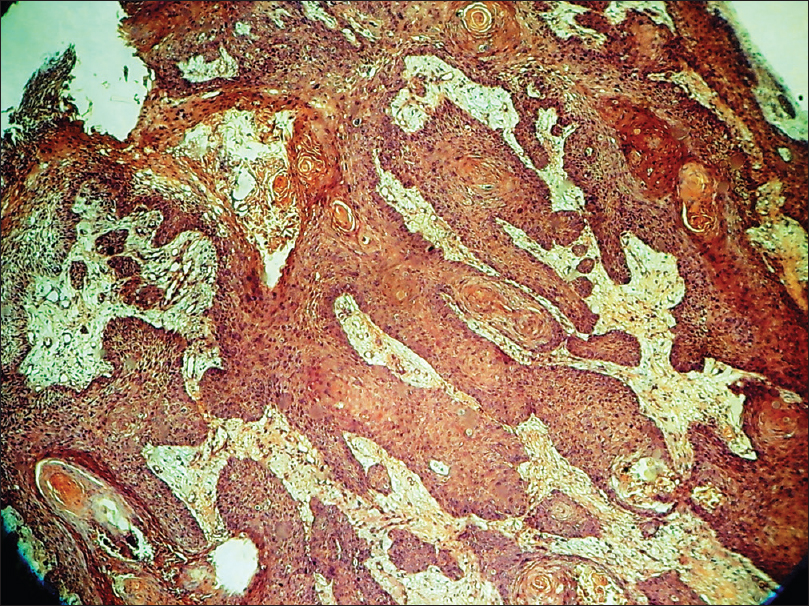 |
| Figure 14c: Squamous cell carcinoma. Histopathology: Pseudo-epitheliomatous hyperplasia, horn pearls formation, multiple mitotic figures and nests of well-differentiated squamous cells in the dermis (H and E, ×100) |
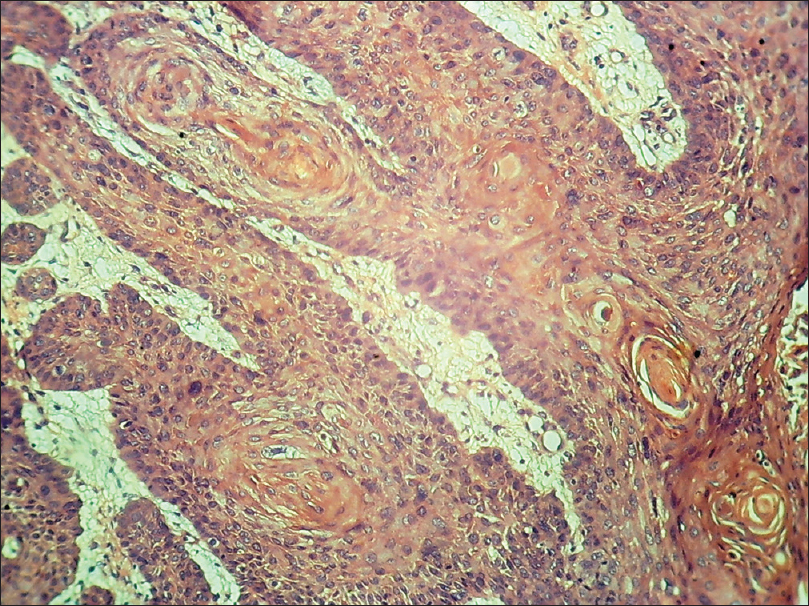 |
| Figure 14d: Squamous cell carcinoma. Histopathology: multiple horn pearls and nests of well-differentiated squamous cells in the dermis (H and E, ×100 and ×400) |
Basal cell carcinoma
Basal cell carcinoma is the most common non-melanocytic skin cancer originating from the basal layer of epidermis.
Clinically, the classical presentation is an ulcerative lesion with rolled out margins. However, nodular or pigmented lesions are commonly encountered [Figure 15a].
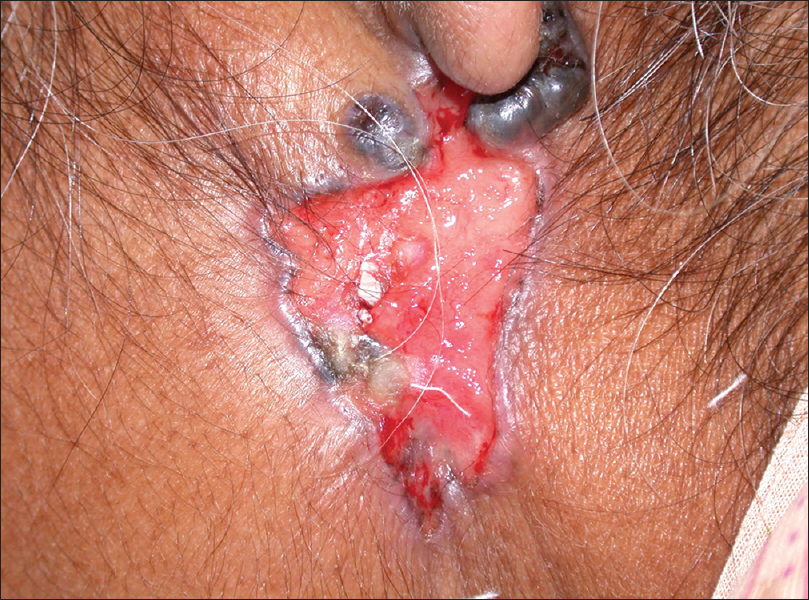 |
| Figure 15a: Basal cell carcinoma. Ulcerative lesion with hyperpigmented, rolled out margins and two hyperpigmented nodules are seen at the upper border |
Ultrasound biomicroscopy of the ulcerated lesions shows an ill-defined, hypoechoic mass in the dermis with irregular margins [Figure 15b]. The pigmented and nodular lesions show a thickened, hyperechoic entry echo with posterior acoustic shadowing [Figure 15c]. The margins of the lesion show an epidermal entry echo with a thin subepidermal hypoechoic band. The underlying dermis shows a non-homogeneous echotexture with an ill-defined dermo-hypodermal junction.
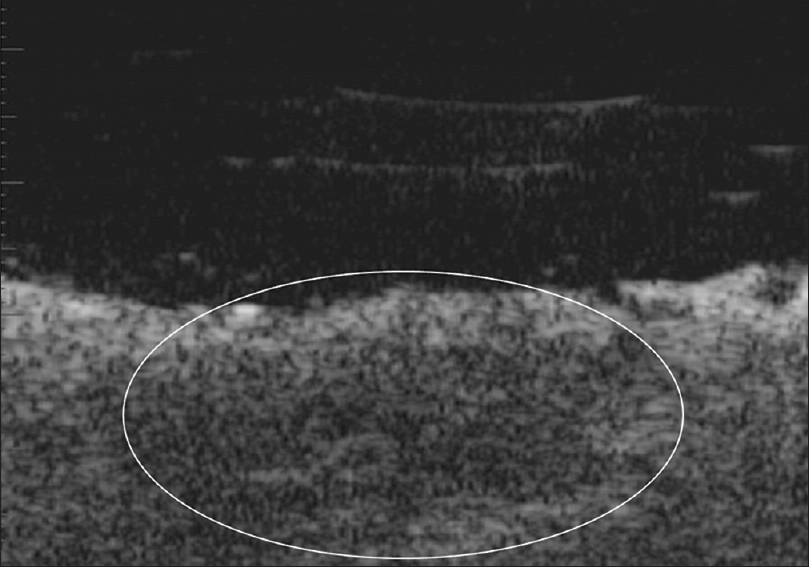 |
| Figure 15b: Basal cell carcinoma. Ultrasound biomicroscopy (50 MHz) of ulcerated lesion showing absence of epidermis and an ill-defined mass lesion in the dermis with irregular margins |
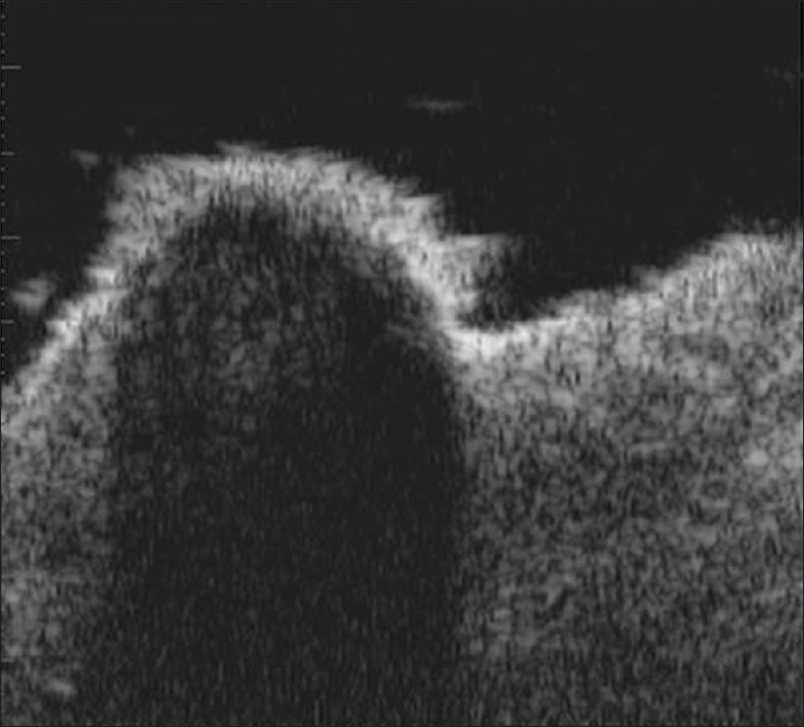 |
| Figure 15c: Basal cell carcinoma. Ultrasound biomicroscopy (50 MHz) of pigmented nodules showing thickened hyperechoic entry echo with posterior acoustic shadowing |
Histopathology shows nests of tumor cells in the dermis originating from the basal layer of epidermis. These basaloid cells are arranged in a peripheral palisading pattern and there are retraction artifacts [Figure 15d].
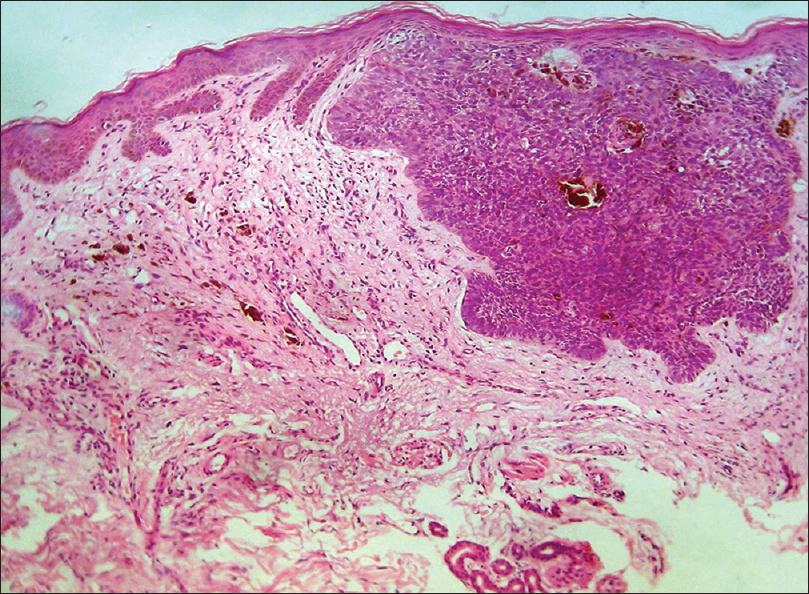 |
| Figure 15d: Basal cell carcinoma. Histopathology: Tumor nests in the dermis arising from the epidermis, composed of basaloid cells with peripheral palisading arrangement and retraction artifacts (H and E, ×100) |
Melanoma
Melanoma is a malignant neoplasm of melanocytes. It should be differentiated from benign melanocytic lesions as well as from the common skin malignancies such as basal cell carcinoma and squamous cell carcinoma.
It usually presents as a flat or nodular, darkly pigmented lesion. Acral lentiginous melanoma is usually seen on the palms, soles and under the nails [Figure 16a] and [Figure 16b].
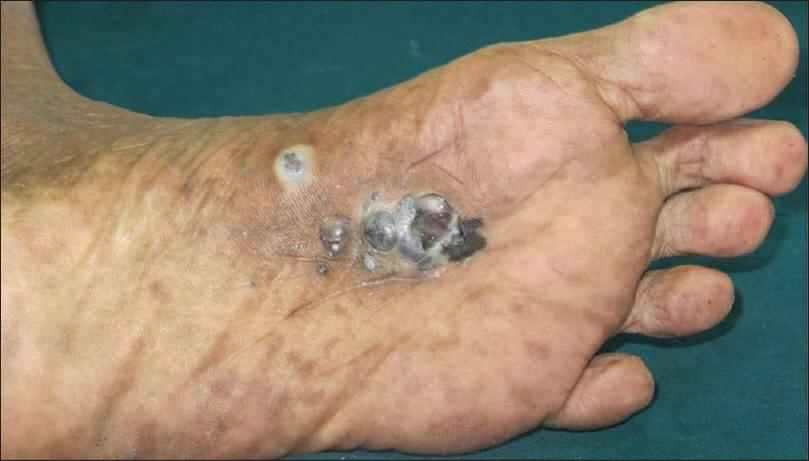 |
| Figure 16a: Melanoma. Multiple black nodular lesions on the sole |
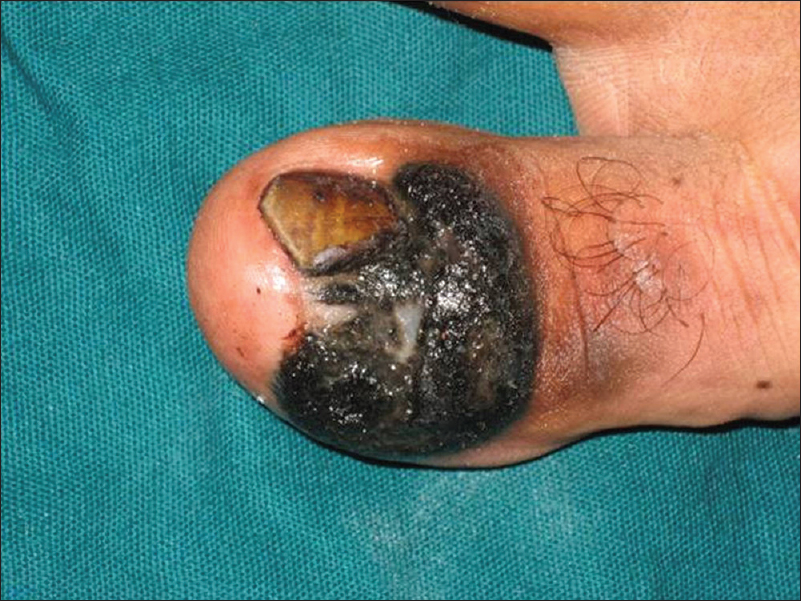 |
| Figure 16b: Melanoma. Periungual and subungual hyperpigmented plaque |
Ultrasound biomicroscopy and high-frequency ultrasonography show well-defined hypoechoic nodular mass lesions in the sub-epidermal, dermal and subcutaneous tissue. The mass lesion measures approximately 2 cm in length and 0.85 cm in depth. On color Doppler study, neovascularity is noted in the mass lesions [Figure 16c] and [Figure 16d].
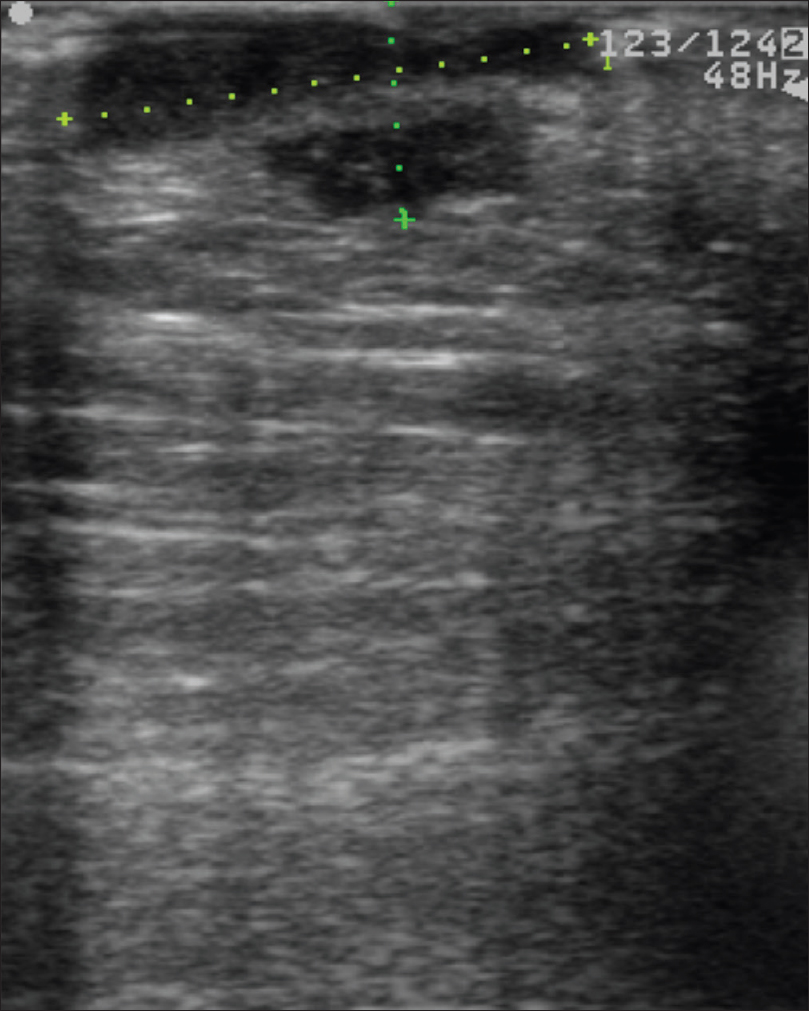 |
| Figure 16c: Melanoma. High-frequency ultrasonography of black nodular lesion on the sole, showing a well-defined hypoechoic nodular mass lesion in the subepidermal, dermal and subcutaneous tissue |
 |
| Figure 16d: Melanoma. Colour doppler study of black nodular lesion on the sole showing neovascularity within the nodular lesion |
Color Doppler study of subungual melanoma shows a thickened epidermal entry echo with a sub-epidermal hypoechoic band and an irregular mass lesion infiltrating the dermis and subcutaneous tissue with neovascularity [Figure 16e] and [Figure 16f]. Sometimes, ultrasonography cannot resolve these mass lesions because of the posterior acoustic shadow caused by the thickened epidermis.
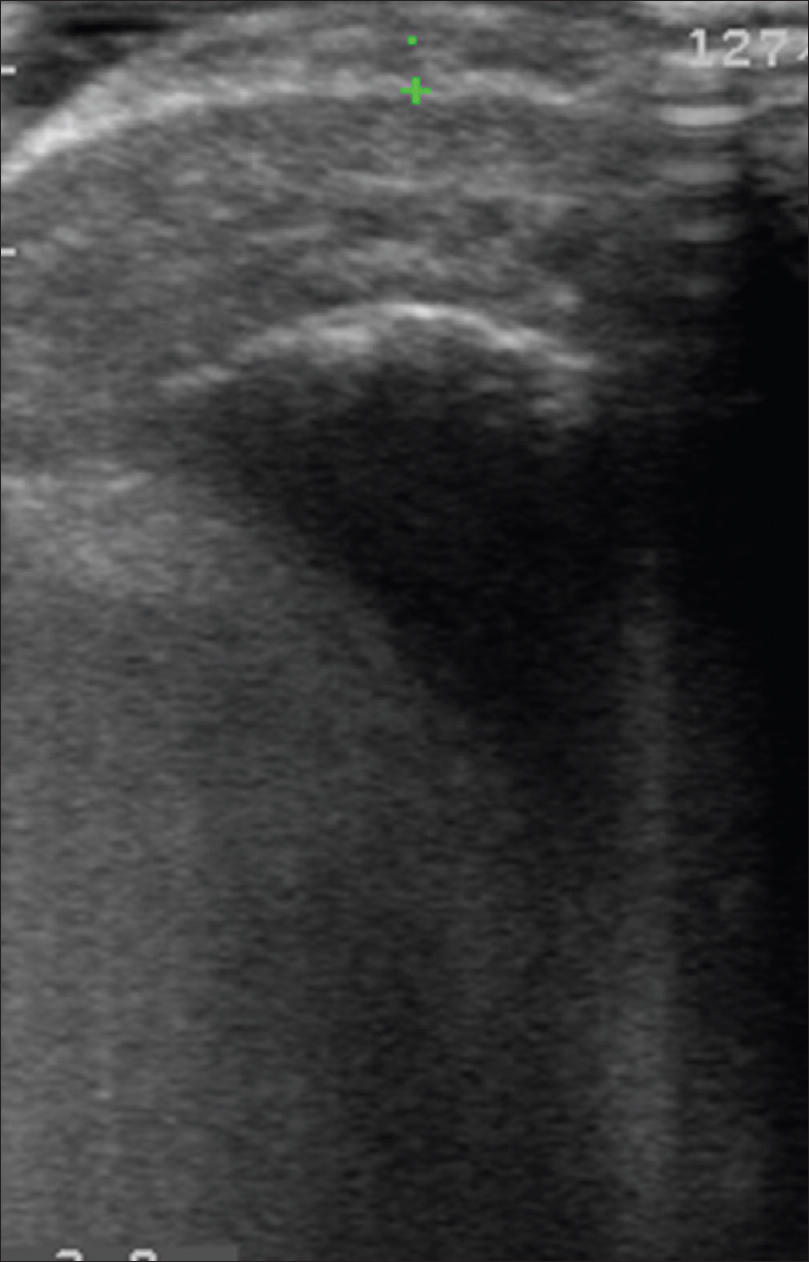 |
| Figure 16e: Melanoma. High-frequency ultrasonography of subungual melanoma showed a thickened entry echo and subepidermal hypoechoic band with irregular mass lesion infiltrating the dermis and subcutaneous tissue |
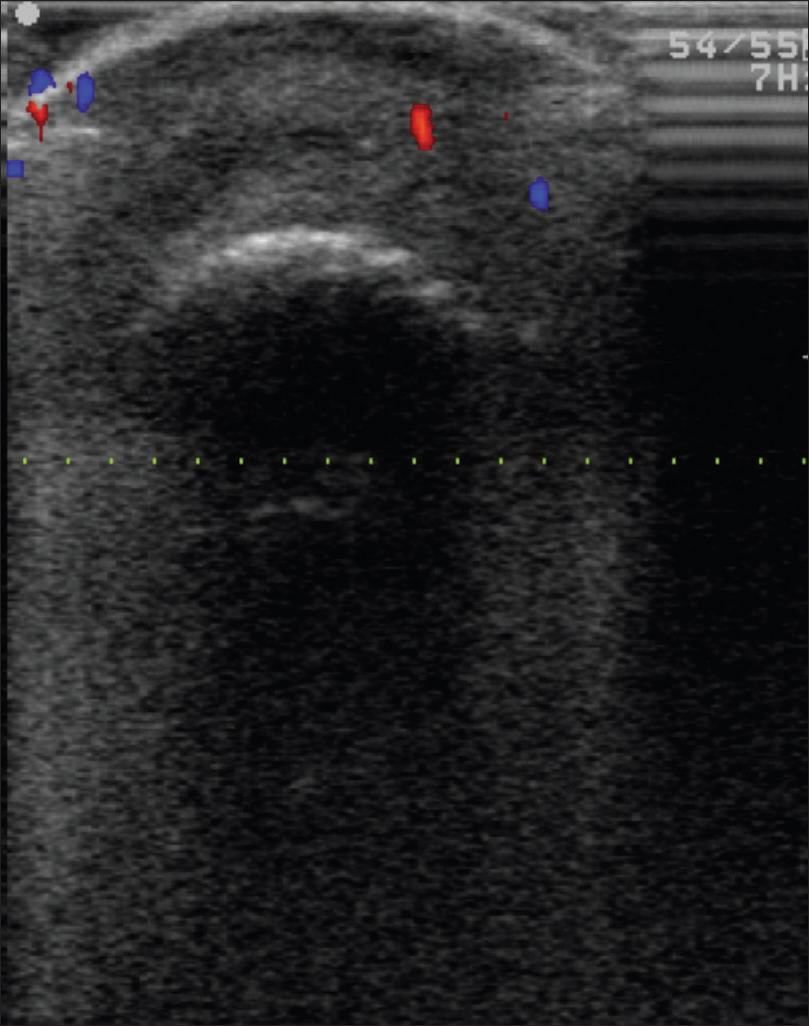 |
| Figure 16f: Melanoma. Colour doppler study of the same lesion showing neovascularity |
Histopathology shows compact hyperkeratosis and diffuse infiltration of the dermis with atypical round melanocytes; focal pigmentary incontinence is present [Figure 16g] and [Figure 16h].
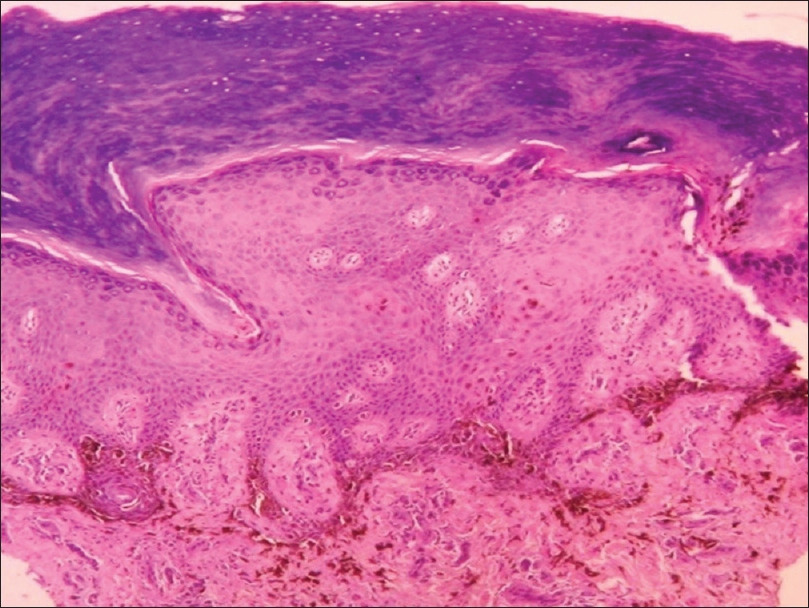 |
| Figure 16g: Melanoma. Histopathology: Compact hyperkeratosis with diffuse infiltration of dermis with atypical melanocytes (H and E, ×100) |
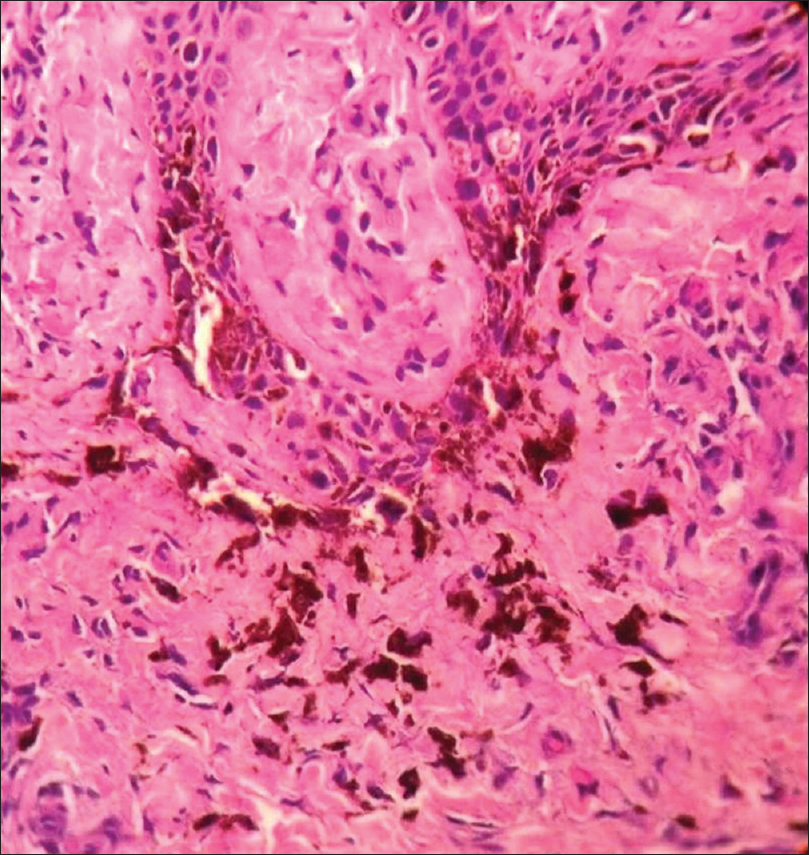 |
| Figure 16h: Melanoma. Histopathology: Multiple atypical large melanocytes in the lower layer of epidermis and in the upper dermis (H and E, ×400) |
Mucinous carcinoma
Mucinous eccrine carcinoma is a rare slow-growing malignant tumor of eccrine sweat glands predominantly involving the periorbital region. It has a varied presentation, often mimicking other benign tumors leading to diagnostic delay.
The usual presentation is a small, solitary, asymptomatic papule or nodule that has been present for several years [Figure 17a].
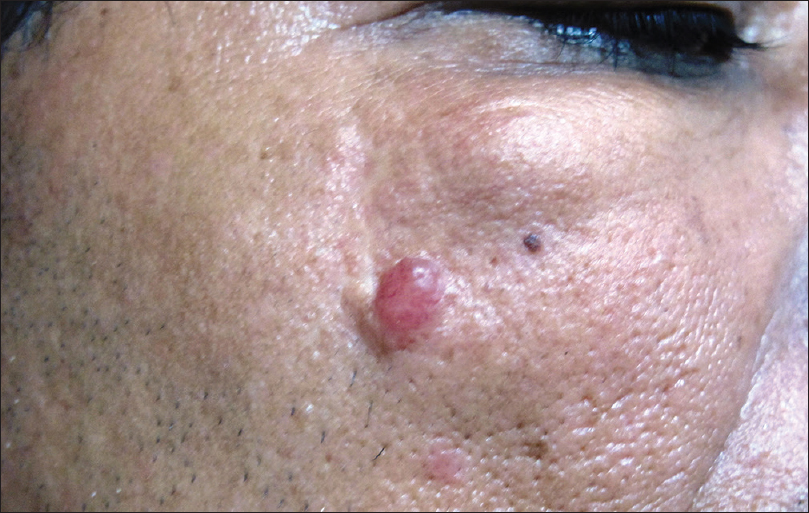 |
| Figure 17a: Mucinous carcinoma. Mobile skin-colored, subcutaneous nodule (3 × 2 cms) just below the right lower eyelid and a translucent reddish fixed nodule (1 cm diameter) on the right cheek, surrounded by a collarette of thickened skin |
High-frequency ultrasonography shows an ill-defined, hypoechoic mass lesion involving the epidermis, dermis, subcutis and underlying muscle. On color Doppler study, neovascularity may be visualized [Figure 17b] and [Figure 17c].
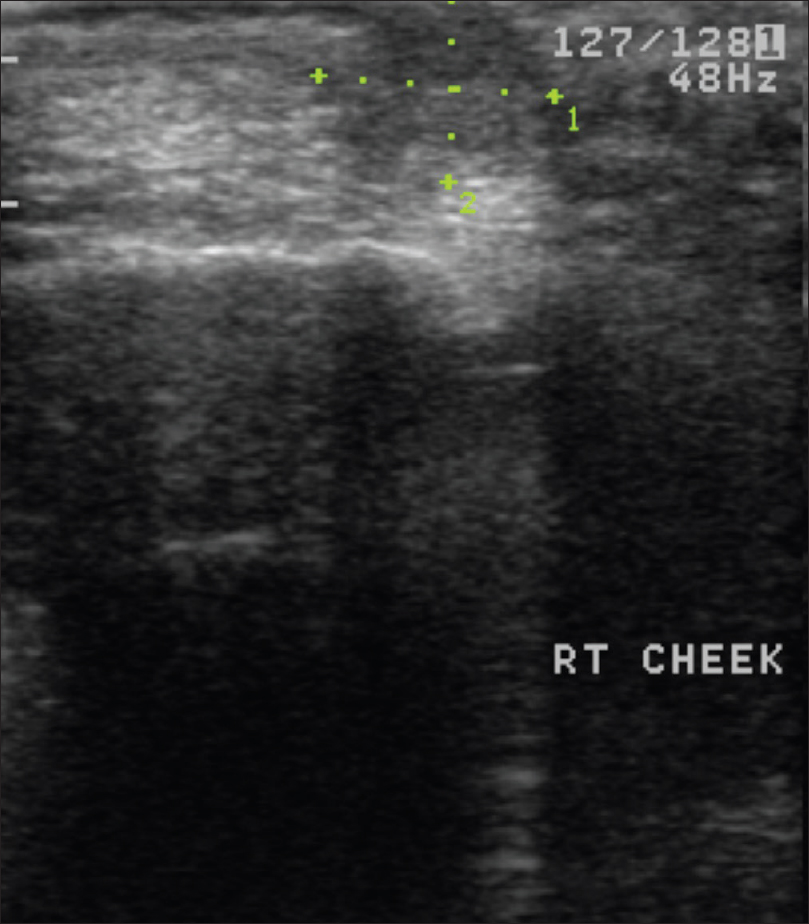 |
| Figure 17b: Mucinous carcinoma. High-frequency ultrasonography: Ill-defined hypoechoic mass lesion involving epidermis, dermis and subcutis |
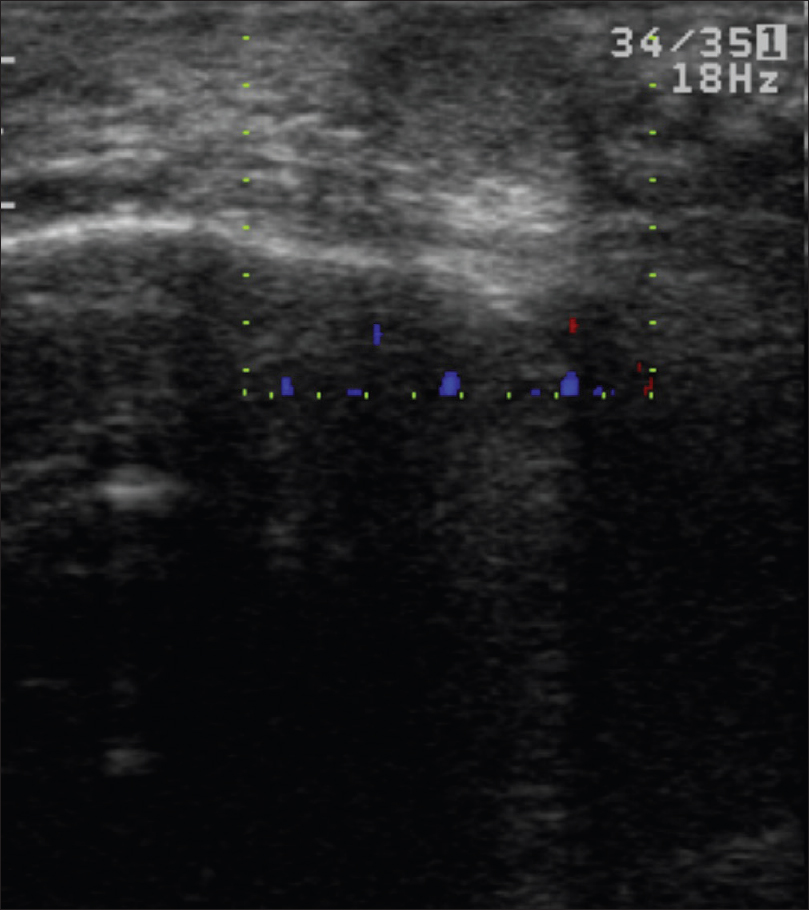 |
| Figure 17c: Mucinous carcinoma. Color Doppler study showed no evidence of neovascularity |
Histopathology shows a tumor mass in the dermis which is divided into numerous compartments by strands of fibrous tissue. Each compartment contains abundant mucin surrounded by nests or cords of anaplastic epithelial cells [Figure 17d].
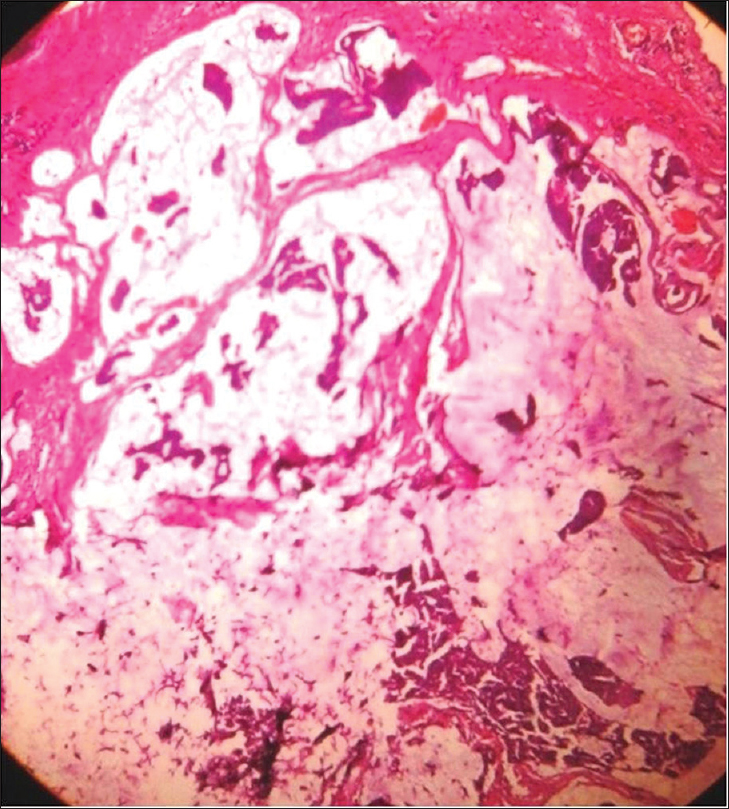 |
| Figure 17d: Mucinous carcinoma. Histopathology: Tumor mass with numerous compartments in the dermis divided by strands of fibrous tissue. Each compartment showed nests of anaplastic epithelial cells with abundant mucin (H and E, ×100) |
Clear cell eccrine hidradenocarcinoma
It is a rare carcinoma of eccrine sweat glands with potential for local destruction, metastasis and high recurrence rates following surgical excision. It usually presents as a single, asymptomatic, slow-growing plaque or nodule [Figure 18a].
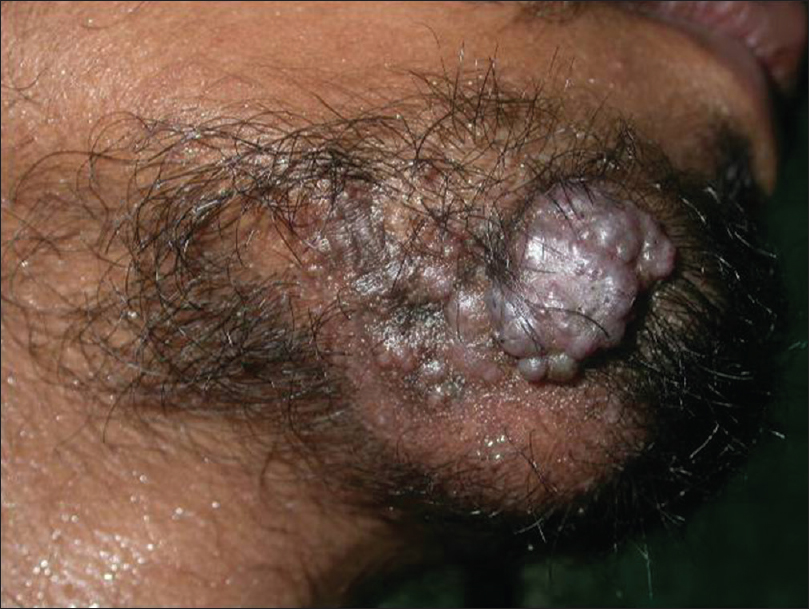 |
| Figure 18a: Clear cell eccrine hidradenocarcinoma. Ill-defined indurated plaque on the chin with multiple verrucous nodules on the surface |
High-frequency ultrasonography shows an ill-defined hypoechoic mass lesion in the epidermis, dermis and subcutaneous tissue extending into the underlying muscle or floor of the mouth. Color Doppler study shows neovascularity in the mass lesion [Figure 18b] and [Figure 18c]. Computed tomography scan is further confirmatory of these findings.
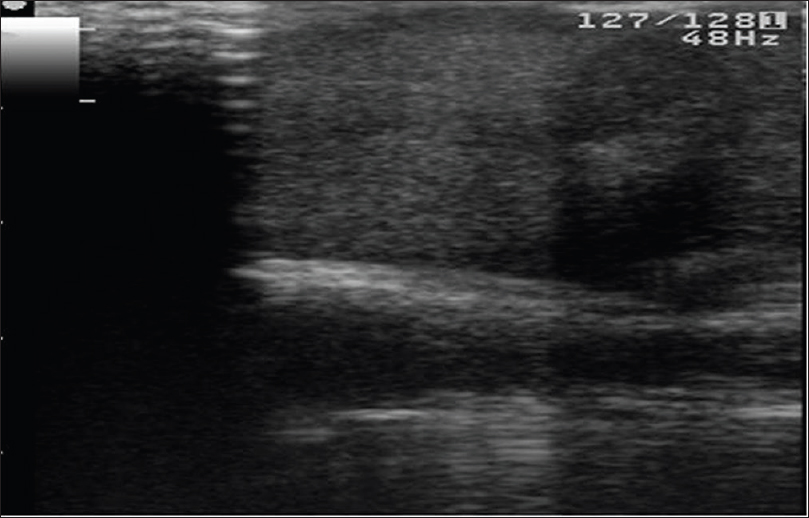 |
| Figure 18b: Clear cell eccrine hidradenocarcinoma. High-frequency ultrasonography: Ill-defined hypoechoic mass lesion in the epidermis, dermis and subcutaneous tissue extending into the underlying muscle |
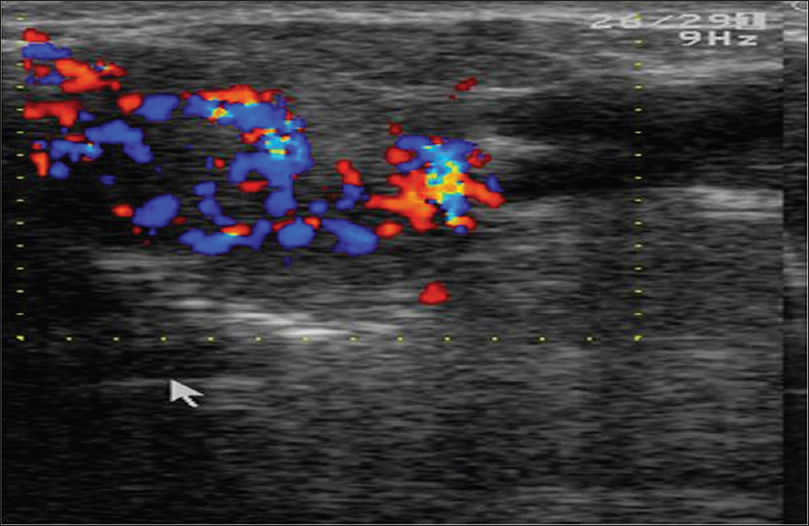 |
| Figure 18c: Clear cell eccrine hidradenocarcinoma. Color Doppler study showing neovascularity in the mass lesion suggesting the malignant nature of the tumor |
Histopathology shows dermal infiltration with lobules and nests of various sizes and cyst formation; the lobules are composed of a mixture of eosinophilic polygonal cells, squamous cells, mucinous and clear cells with pleomorphism and necrosis [Figure 18d] and [Figure 18e].
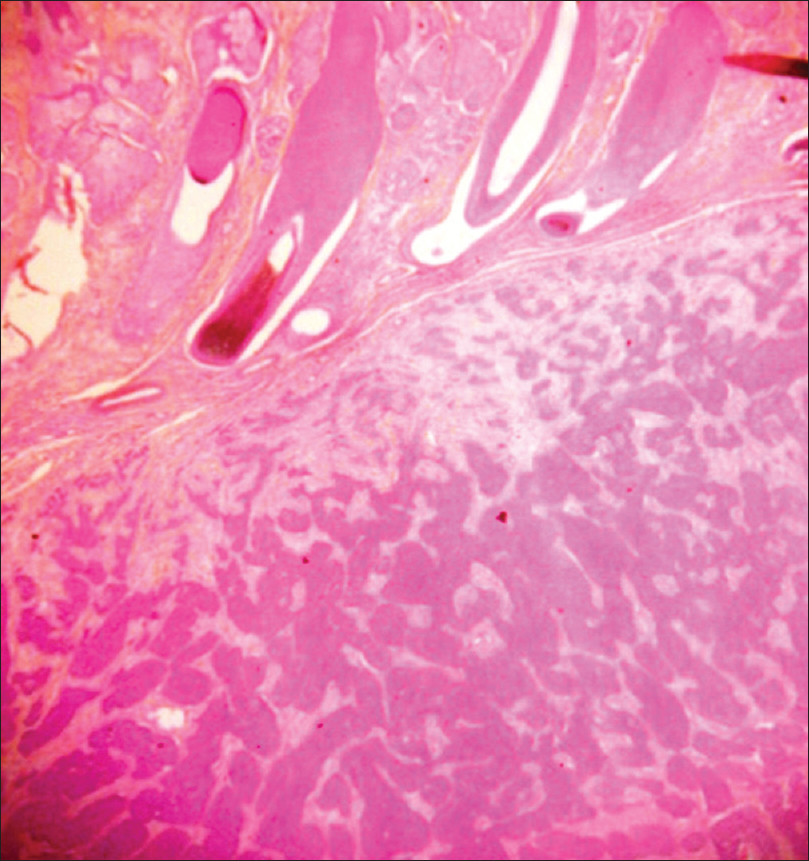 |
| Figure 18d: Clear cell eccrine hidradenocarcinoma. Histopathology: Infiltrative dermal proliferation of lobules and nests (H and E, ×100) |
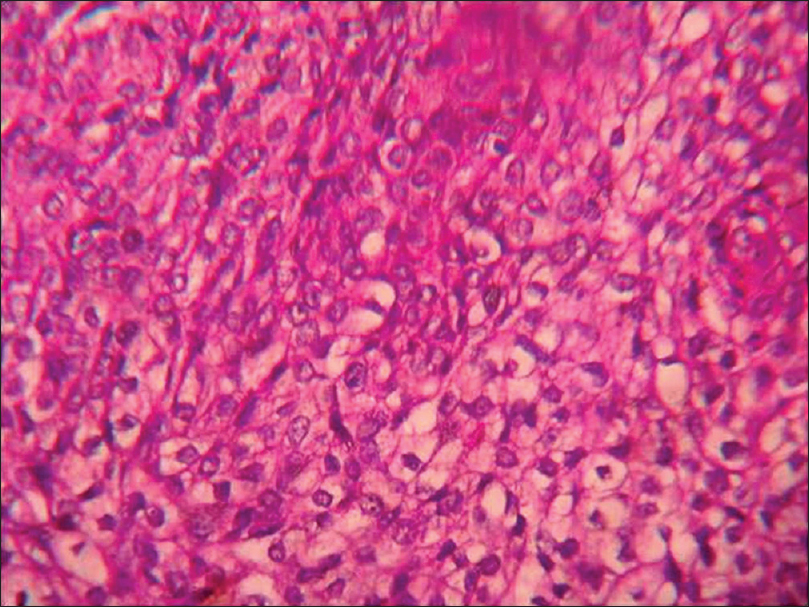 |
| Figure 18e: Clear cell eccrine hidradenocarcinoma. Tumor composed of mixture of eosinophilic polygonal cells, squamous cells, mucinous and clear cells with pleomorphism and necrosis is seen on higher magnification (H and E, ×400) |
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma protuberans is a mesenchymal tumor of dermis and subcutaneous tissue presenting as a sharply defined plaque with nodularity on the surface [Figure 19a]. It is commonly misdiagnosed as keloid.
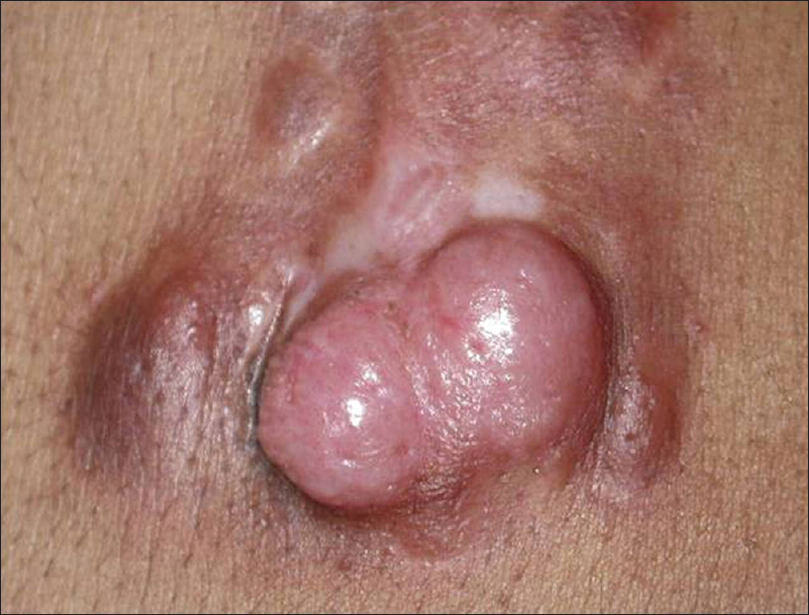 |
| Figure 19a: Dermatofibrosarcoma protuberans. Well-defined plaque with nodules on the surface resembling keloids |
Ultrasound biomicroscopy and high-frequency ultrasonography show an ill-defined, mixed echogenic mass lesion arising from the dermis and extending into the subcutaneous tissue [Figure 19b] and [Figure 19c]. On color Doppler study, minimal vascularity is noted in the mass lesion [Figure 19d].
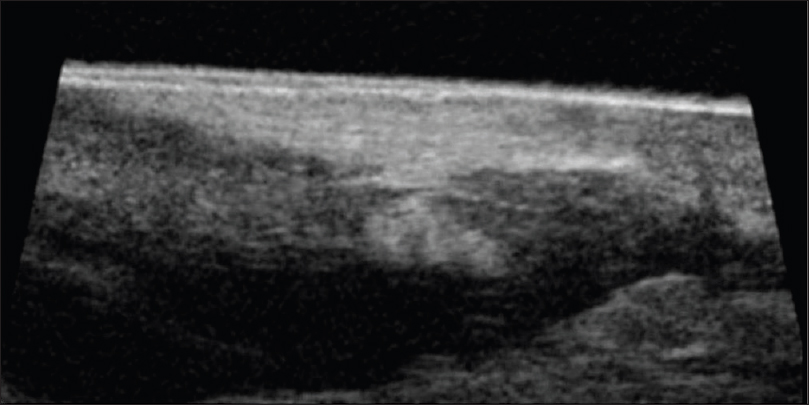 |
| Figure 19b: Dermatofibrosarcoma protuberans. Ultrasound biomicroscopy (50 MHZ): Showing a mass lesion in the dermis extending into the subcutaneous tissue. The margins of the tumor are appearing well defined except at one end where the demarcation between normal and abnormal tissue is not seen |
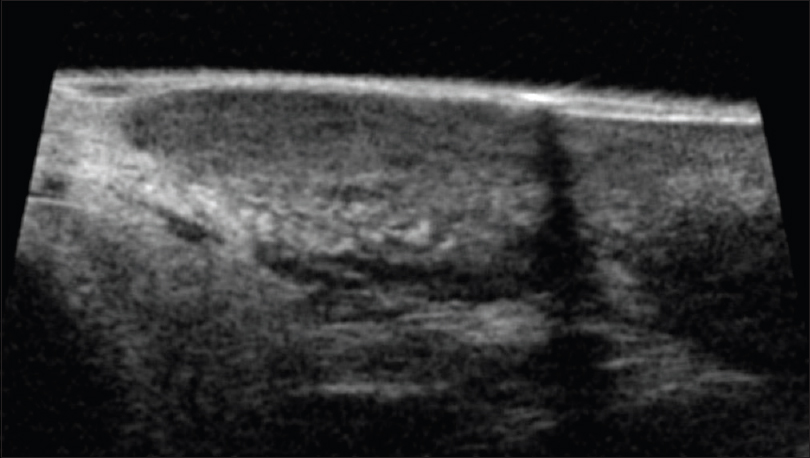 |
| Figure 19c: Dermatofibrosarcoma protuberans. Ultrasound biomicroscopy (50 MHZ showing a mixed echogenic mass lesion in the dermis extending into the subcutaneous tissue with well defined margins |
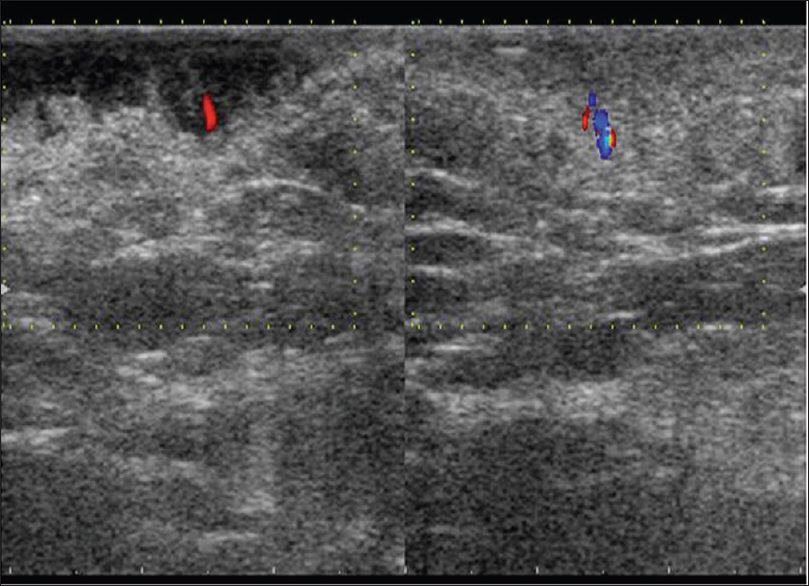 |
| Figure 19d: Dermatofibrosarcoma protuberans. Color Doppler study showing increased vascularity in the subcutaneous region |
Histopathology shows storiform proliferation of monotonous slender spindle cells with dark nuclei. Diffuse infiltration of these cells into the subcutaneous tissue gives a honey-comb appearance [Figure 19e] and [Figure 19f].
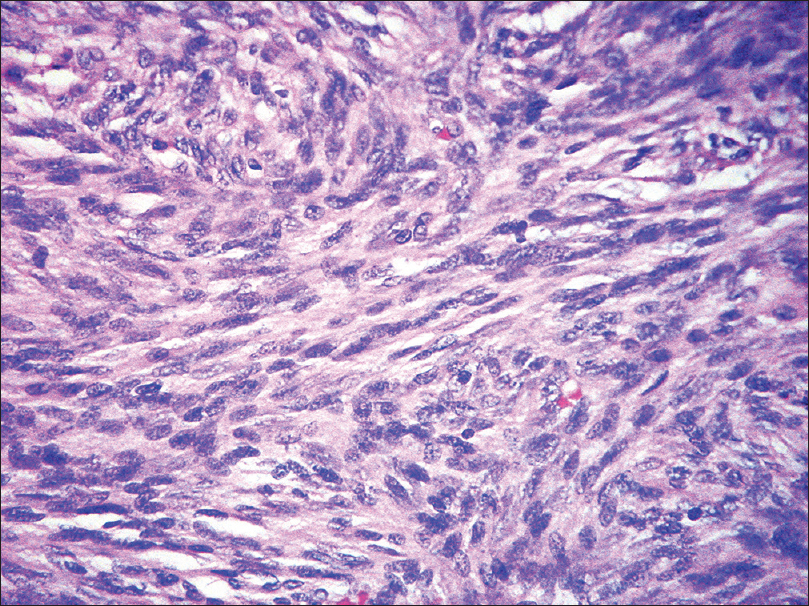 |
| Figure 19e: Dermatofibrosarcoma protuberans. Histopathology: Storiform proliferation of monomorphous slender spindle cells with dark nuclei (H and E, ×400) |
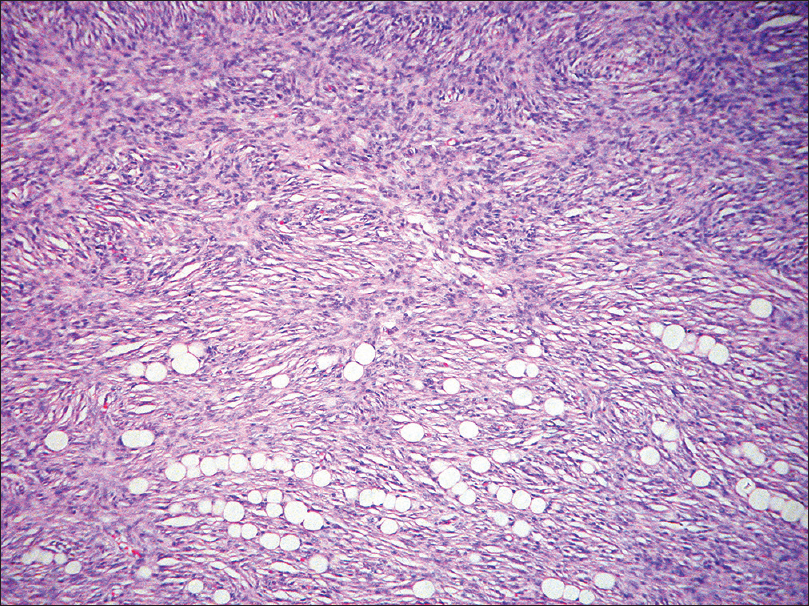 |
| Figure 19f: Dermatofibrosarcoma protuberans. Diffuse infiltration of the cells into the subcutaneous tissue giving rise to a honey-comb appearance (H and E, ×100) |
As the tumor has high chances of recurrence, regular monitoring is a must. High-resolution ultrasonography is extremely important to detect early post-operative recurrence.
Cutaneous T-cell lymphoma (mycosis fungoides)
Cutaneous T-cell lymphoma is a group of lymphoproliferative disorders characterized by cutaneous infiltration by neoplastic T lymphocytes. It may present with variable morphology such as patches, plaques, nodules and erythroderma, with or without lymphadenopathy [Figure 20a].
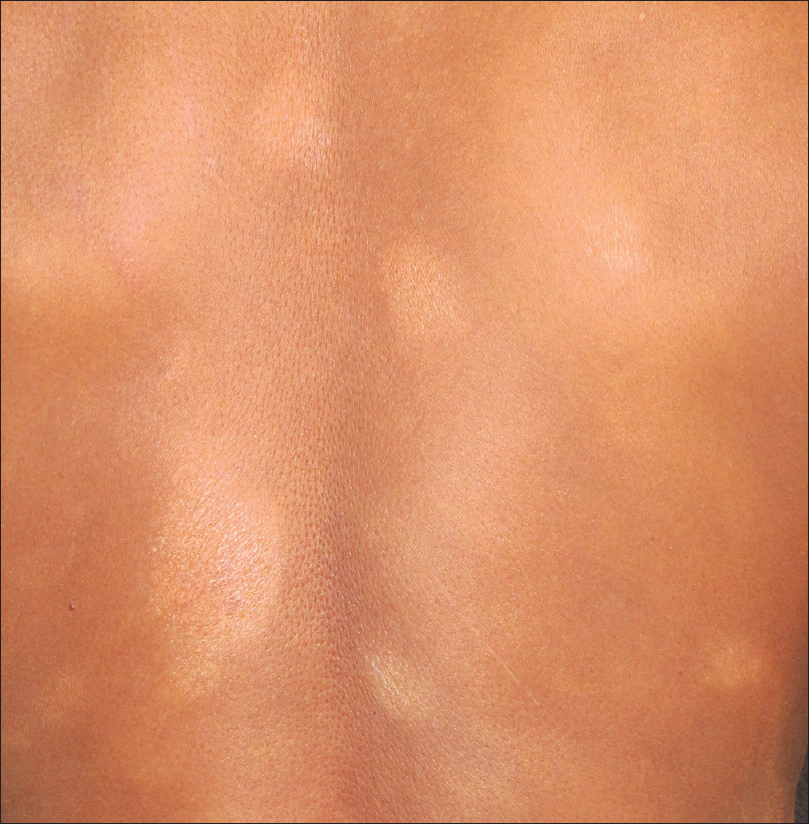 |
| Figure 20a: Cutaneous T-cell lymphoma. Multiple Hypopigmented scaly plaques on the back |
Ultrasound biomicroscopy shows a thickened, hyperechoic epidermal entry echo with a thick, subepidermal hypoechoic band [Figure 20b] and [Figure 20c].
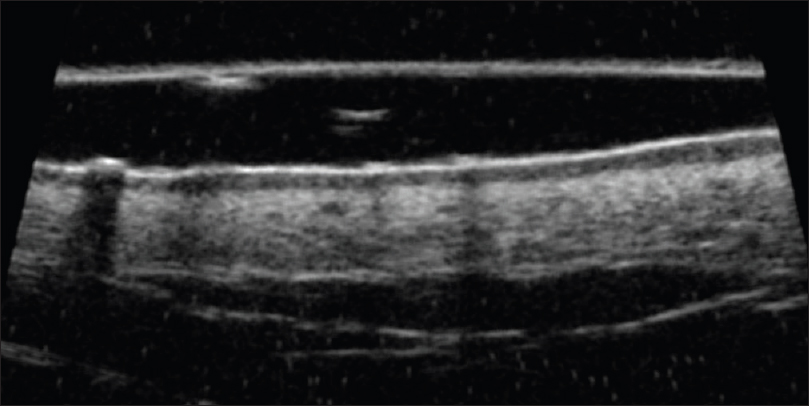 |
| Figure 20b: Cutaneous T-cell lymphoma. Ultrasound biomicroscopy (35 MHz) showing a thickened hyperechoic epidermal entry echo with a subepidermal hypoechoic band with inhomogenous dermal echotexture |
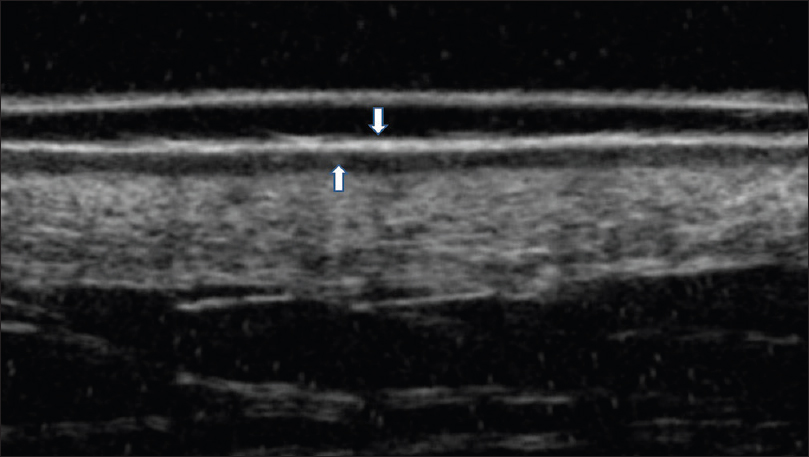 |
| Figure 20c: Cutaneous T-cell lymphoma. Ultrasound biomicroscopy (35 MHz) showing a thickened hyperechoic epidermal entry echo with a subepidermal hypoechoic band |
Histopathology shows a band-like upper dermal infiltrate of lymphocytes and other inflammatory cells without any Grenz zone. There is epidermotropism of mononuclear cells and Pautrier's microabscesses are seen. There is mild spongiosis of the epidermis. Lymphocytes show hyperchromatic and convoluted or cerebriform nuclei [Figure 20d].
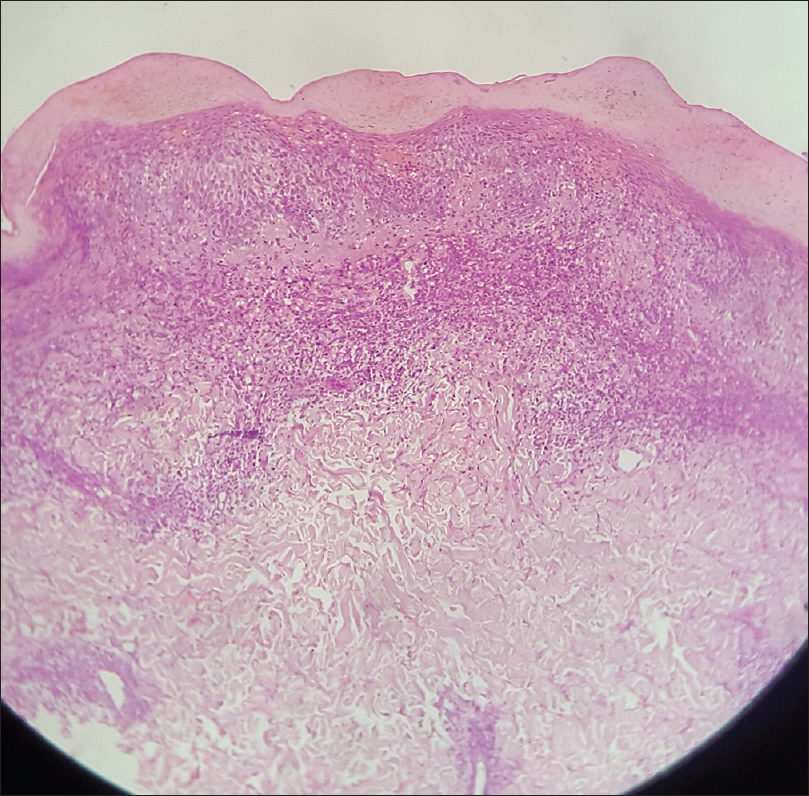 |
| Figure 20d: Cutaneous T-cell lymphoma. Histopathology: Band-like upper dermal infiltrate of lymphocytes and other inflammatory cells, without a Grenz zone. Epidermotropism of mononuclear cells and mild spongiosis of the epidermis is noted. Lymphocytes show hyperchromatic and convoluted or cerebriform nuclei (H and E, ×100) |
Advantages and Limitations
Since ultrasonography is a non-invasive diagnostic modality, patient compliance is excellent. Tumors as small as 50 µ can be detected by ultrasound biomicroscopy. Important characteristics of a tumor can be visualized in situ and multiple tumor sites can be scanned during a single visit.
The major limitation of this modality is that it cannot provide a tissue diagnosis. Differentiation between tumoral and inflammatory infiltrate is not possible, this may result in over-estimation of the dimensions of a tumor.[15]
Though biopsy is the gold standard for diagnosis of skin tumors, in larger and heterogenous lesions its use may be limited. Desired depth of the tumor may not be reached, all tissue variants may not be accessible by a single biopsy and multiple biopsies may be required for co-existent lesions of different morphology. Hence, there is a definite need for an objective, non-invasive modality for the diagnosis and localization of skin tumors. High frequency ultrasonography serves this purpose as it may provide reasonably accurate information on tumor characteristics such as size, shape, depth, consistency and vascularity before performing invasive skin biopsy or surgery. It has also been found to be a helpful modality for early detection of tumor recurrence during the post-operative period. Further development of this technique in the diagnosis of skin tumors is required. An ultrasonography probe with color and pulsed Doppler and 100 MHz or 1 GHz probe has to be designed for studying epidermal pathologies exclusively.[16] High-frequency ultrasonography with contrast (commonly used in hepatic tumors) would be a useful additional tool to differentiate malignant and benign skin tumors.
Acknowledgment
Dr. Chitra Nayak, Professor and Head, Department of Dermatology, BYL Nair Hospital, Mumbai, for access to the photomicrographs and contributing photomicrographs of seborrheic keratosis and mycosis fungoides.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Serup J. Ten years experience with high frequency ultrasound examination of the skin. In: Hoffman K, editor. Ultrasound in Dermatology. 1st ed. Germany: Springer-Verlag; 1992. p. 41-54.
[Google Scholar]
|
| 2. |
Schmid-Wendtner MH, Dill-Müller D. Ultrasound technology in dermatology. Semin Cutan Med Surg 2008;27:44-51.
[Google Scholar]
|
| 3. |
Nessi R, Blanc M, Bosco M, Dameno S, Venegoni A, Betti R, et al. Skin ultrasound in dermatologic surgical planning. J Dermatol Surg Oncol 1991;17:38-43.
[Google Scholar]
|
| 4. |
Hoffmann K, El Gammal S, Winkler K, Jung J, Pistorius K, Altmeyer P. Skin tumours in high frequency ultrasound. In: Altmeyer P, El Gammal S, Hoffmann K, editors. Ultrasound in Dermatology. 1st ed. Heidelberg, Germany: Springer-Verlag; 1992. p. 181-201.
[Google Scholar]
|
| 5. |
Hoffmann K, Happe M, Schüller S, Stücker M, Wiesner M, Gottlöber P, et al. Ranking of 20 MHz sonography of malignant melanoma and pigmented lesions in routine diagnosis. Ultraschall Med 1999;20:104-9.
[Google Scholar]
|
| 6. |
Vassallo P, Edel G, Roos N, Naguib A, Peters PE.In vitro high-resolution ultrasonography of benign and malignant lymph nodes. A sonographic-pathologic correlation. Invest Radiol 1993;28:698-705.
[Google Scholar]
|
| 7. |
Solbiati L, Arsizio B, Rizzatto G, et al. High-resolution sonography of cervical lymph nodes in head and neck cancer: Criteria for differentiation of reactive versus malignant lymph nodes (abstract). Radiology 1998.
[Google Scholar]
|
| 8. |
Garbe C, Hauschild A, Volkenandt M, Schadendorf D, Stolz W, Reinhold U, et al. Evidence-based and interdisciplinary consensus-based German guidelines: Systemic medical treatment of melanoma in the adjuvant and palliative setting. Melanoma Res 2008;18:152-60.
[Google Scholar]
|
| 9. |
Ulrich J, Voit C. Ultrasound in dermatology. Part II. Ultrasound of regional lymph node basins and subcutaneous tumours. Eur J Dermatol 2001;11:73-9.
[Google Scholar]
|
| 10. |
Moehrle M, Blum A, Rassner G, Juenger M. Lymph node metastases of cutaneous melanoma: Diagnosis by B-scan and color Doppler sonography. J Am Acad Dermatol 1999;41 (5 Pt 1):703-9.
[Google Scholar]
|
| 11. |
Tschammler A, Wirkner H, Ott G, Hahn D. Vascular patterns in reactive and malignant lymphadenopathy. Eur Radiol 1996;6:473-80.
[Google Scholar]
|
| 12. |
Tambe S, Someshwar S, Dedhia A, Jadhav R, Bhatt K, Jerajani H. An asymptomatic swelling on the neck. Indian J Dermatol Venereol Leprol 2015;81:221-3.
[Google Scholar]
|
| 13. |
Dedhia A, Tambe S, Jadhav R, Bhatt K, Jerajani H. Unusual location of glomus tumour on the right ring finger. J Cutan Aesthet Surg 2014;7:179-81.
[Google Scholar]
|
| 14. |
Bhatt KD, Fernandes R, Dhurat R. Ultrasound biomicroscopy of the skin to detect a subclinical neuroma of the proximal nail-fold. Indian J Dermatol Venereol Leprol 2006;72:60-2.
[Google Scholar]
|
| 15. |
Mandava A, Konathan R, Neelala K. Utility of high-resolution ultrasonography and colour Doppler in the assessment of pigmented skin lesions. Ultrasound 2012;20:155-60.
[Google Scholar]
|
| 16. |
Serup J. Ten years experience with high frequency ultrasound examination of the skin. In: Hoffman K, editor. Ultrasound in Dermatology. 1st ed. Germany: Springer-Verlag; 1992. p. 399-419.
[Google Scholar]
|
Fulltext Views
14,224
PDF downloads
16,373





