Translate this page into:
Utility of photodynamic diagnosis plus reflectance confocal microscopy in detecting the margins of extramammary Paget disease
Corresponding author: Dr. Liang Zhao, Department of Dermatologic Surgery, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, 12 Jiang-Wang-Miao Road, Nanjing 210042, Jiangsu Province, China. zhaoliang210042@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Huang L, Lu X, Li J, Wu M, Liu X, Zhao L, et al. Utility of photodynamic diagnosis plus reflectance confocal microscopy in detecting the margins of extramammary Paget disease. Indian J Dermatol Venereol Leprol 2021;87:207-13.
Abstract
Background:
Due to the clinically poorly delineated unclear margin of extramammary Paget disease, the recurrence rate after surgical resection is high.
Aims:
To compare photodynamic diagnosis and photodynamic plus reflectance confocal microscopy diagnosis in determining the tumor margins in patients with extramammary Paget disease.
Methods:
Thirty-six patients with histopathologically confirmed primary extramammary Paget disease between January 2017 to June 2018 were included in the study. The skin lesion margins were preoperatively observed by the naked eye and with photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy and they were compared to the postoperative histopathological examination results.
Results:
Among the 130 sections taken from 36 patients, 83 sections (63.8%, 83/130) had tumor margins beyond the macroscopic line with a distance of 3.5 ± 3.1mm and a median of 2.7mm. Forty-six sections (35.4%, 46/130) exceeded the photodynamic diagnosis marker line with a distance of 2.1 ± 1.7mm and a median of 1.5mm. Twenty seven sections (20.8%, 27/130) were obtained beyond the photodynamic diagnosis plus reflectance confocal microscopy marker line with a distance of 1.4 ± 1.2mm and a median of 0.9mm.
Limitations:
Photodynamic diagnosis and reflectance confocal microscopy detection can be used to observe only the superficial margin of the tumor and not the deep part. Moreover, reflectance confocal microscopy was not used alone as a control.
Conclusion:
In terms of determining the extramammary Paget disease margin invasively, photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy were found superior to observations made with the naked eye, while photodynamic diagnosis plus reflectance confocal microscopy was superior to photodynamic diagnosis alone.
Keywords
Extramammary Paget disease
fluorescence
margin
photodynamic diagnosis
reflectance confocal microscopy
Introduction
Extramammary Paget disease is a rare malignant skin tumor. Although Mohs surgery has significant advantages in identifying tumor resection edges and preserving normal tissues, it is time-consuming, expensive and requires professional, specially trained dermatologists and pathologists to operate together.1 Local expanded surgical resection remains the preferred treatment for patients with extramammary Paget disease.2 However, due to the clinically poorly delineated margins of extramammary Paget disease and the depth of tumor cell invasion, the surgical results are usually not satisfactory with a recurrence rate of 20% to 60%.3 Photodynamic diagnosis and reflectance confocal microscopy examination have been shown to be efficacious in tumor diagnoses.4 This study aims to determine the value of these three methods (examination with naked eye, photodynamic diagnosis and photodynamic diagnosis with additional confocal microscopy) in determining the tumor margins and comparing it with the actual distance between the tumor margin as assessed in histopathologic sections.
Methods
Patient selection
This study included 36 patients who were histo-pathologically diagnosed with primary extramammary Paget disease from January 2017 to June 2018. Patients with secondary extramammary Paget disease and those who could not undergo surgery or refused surgery were excluded. This study was approved by the Institutional Ethics Committee of the Institute of Dermatology, the Chinese Academy of Medical Sciences and The Fifth People’s Hospital of Suzhou. All patients provided signed informed consent.
Pretreatment of skin lesions
Exudate or erosion can decrease fluorescence. To minimize this interference in patients with erosive exudation, a wet compress was applied using a 3% boric acid solution and zinc oxide was applied topically three times daily. Treatment was administered until the exudates on the surface of the skin lesions disappeared and the redness and swelling significantly improved.
Methods
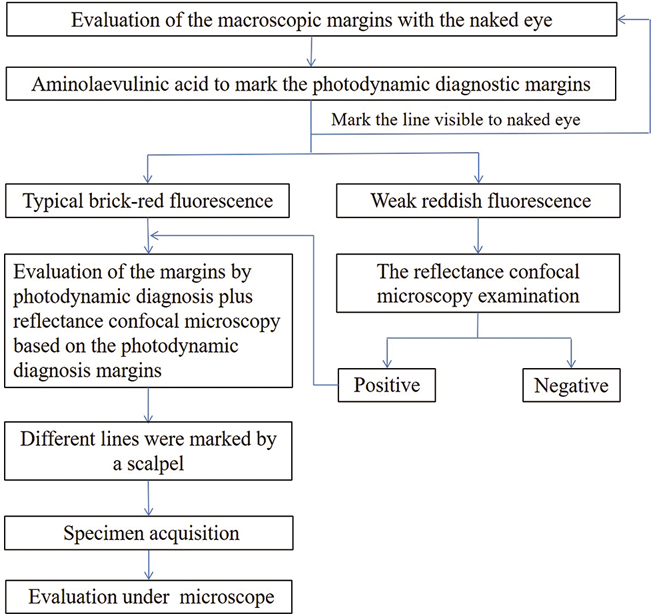
The margins of the skin lesions were observed by the naked eye, photodynamic diagnosis and reflectance confocal microscopy, as follows:
Evaluation of the macroscopic margins with the naked eye
Under normal light, the scope extent of the skin lesions visible to the naked eye was observed [Figure 1a].

- Extramammary Paget disease lesions. Infiltrative erythema (7.6cm × 8.5cm) on the pubic mound, the penis, scrotum
Aminolaevulinic
acid to mark the photodynamic diagnostic margins Aminolaevulinic acid hydrochloride was diluted in water for injection at a concentration of 20%. A thin sheet of makeup cotton was used to cover the skin lesion 5 cm beyond the macroscopic margin. The makeup cotton was evenly dripped and soaked with a 20% aminolaevulinic acid solution. A sheet of tinfoil was applied for further covering and adhesive tape was used for fixation. After 2 h, the patient was transferred to a dark room and the covering on the surface of the skin lesion was removed. A typical lesion under the Wood’s lamp showed brick-red fluorescence. A marker pen was used to mark the scope extent of the fluorescence.The area showing weak reddish fluorescence was marked at the same time[Figure 1b].Then, under normal light, the margin of the lesions visible to the naked eye was marked. If the weak fluorescence region was confirmed with a reflectance confocal microscope, the weak fluorescence marker line was considered to be the photodynamic diagnostic marker line. Otherwise, the brick-red fluorescence region marker line was considered to be the photodynamic diagnostic marker line.
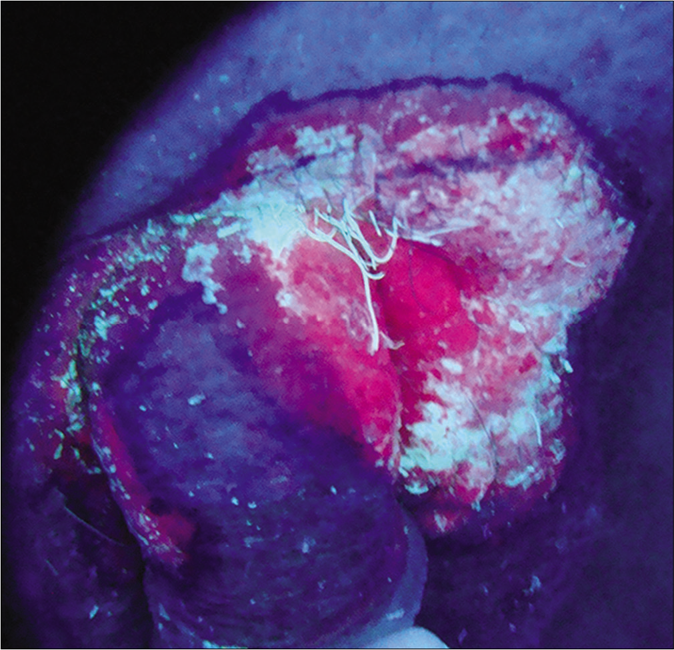
- Photodynamic diagnosis and photodynamic diagnostic line. Brick- red fluorescence was observed on the pubic mound, penile, upper scrotum under Wood’s light. The photodynamic fluorescence areas were delineated, including weak fluorescence with a width of 0.2–0.5cm off the brick- red fluorescence areas, the left pubic mound
Evaluation of the margins by photodynamic diagnosis plus reflectance confocal microscopy based on the photodynamic diagnosis margins
The skin lesions were then examined using a Vivascope 1500 (wavelength 830nm) confocal microscope (Lucid Inc., Rochester, NY, USA) along the photodynamic diagnosis marker line. When tumor cells were detected under the microscope [Figure 1c], the reflectance confocal microscopy examination was expanded 0.5 cm outward until no more tumor cells were found. Then, the photodynamic diagnosis plus reflectance confocal microscopy detection line was marked [Figure 1d].
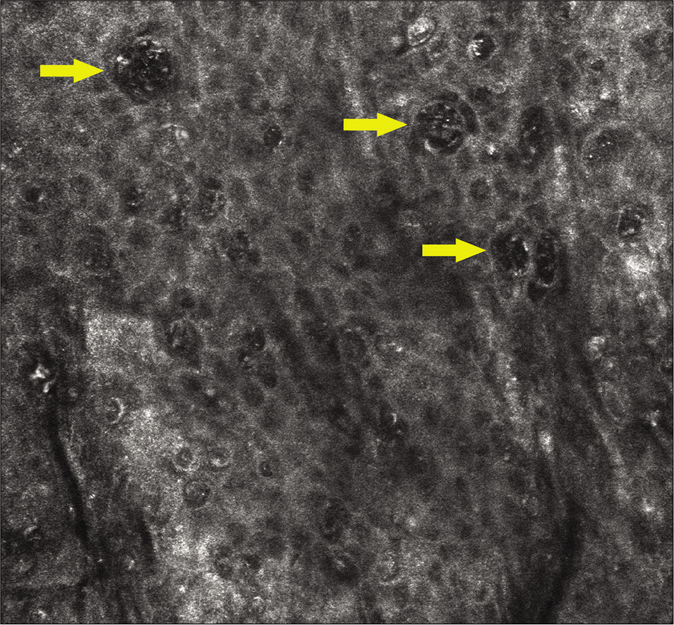
- Extramammary Paget disease characterized by highly refractive, large-nucleated cells on the epidermis under reflectance confocal microscopy. The yellow arrows represent the Paget cells
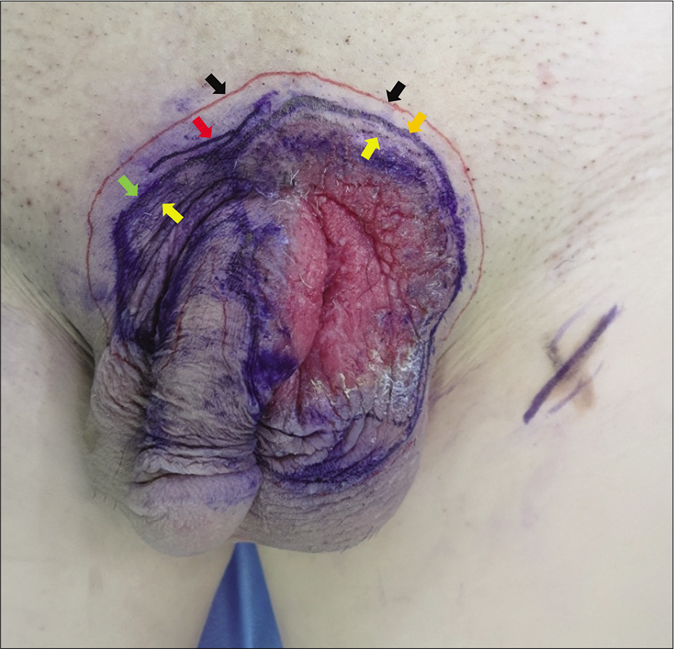
- Multiple marker lines on the lesion surface. Colored arrows: Yellow-macroscopic line; Green-photodynamic diagnostic line; Red-photodynamic diagnosis plus reflectance confocal microscopy diagnostic line; Black-surgical resection line; Orange-the overlapping line composed of photodynamic diagnostic line and photodynamic diagnosis plus reflectance confocal microscopy diagnostic line
Different lines were marked by a scalpel
The surgical resection line was marked by expanding 0.5–2.0 cm outward from the outermost marker line. The specific distance was determined by the surgeon. Under local, intravertebral or general anesthesia, the epidermis was cut using a scalpel, along the marker lines of the photodynamic diagnosis plus reflectance confocal microscopy, the photodynamic fluorescence diagnosis and the clinical macroscopic skin lesion mark which was taken as the marker during the histopathological examination [Figure 1e]. The skin lesion was resected along the surgical excision marker line. A skin flap or skin graft was used to repair the wound.
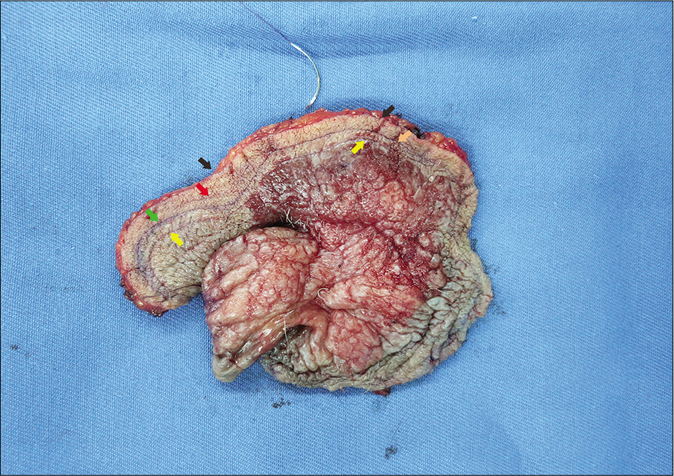
- Excised tumor tissue. The tumor surface was incised by a scalpel along each marker line. The color arrows legends are the same as Figure 1c
Specimen acquisition
The edges of each resected specimen were cut in 1 to 2 places according to different anatomical positions. If the skin lesion was located at the same anatomical site, four specimens were cut in the direction of 12, 3, 6 and 9 o’clock positions [Figure 1f]. The specimen was cut from the visible skin lesion to the surgical resection margin, including three marker lines that were visible to the naked eye and examined by photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy, respectively. The width of the specimens was about 0.5cm and the specific resection site was recorded.
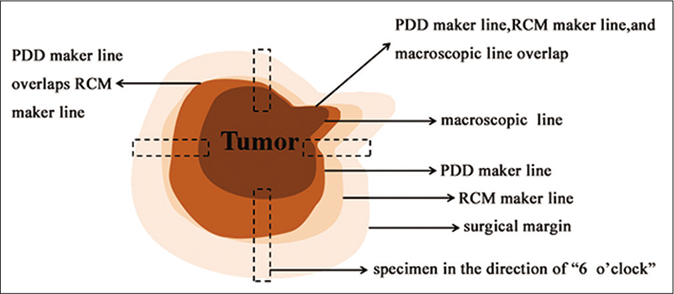
- Schematic diagram of tumor tissue and each diagnostic line. The edges of each resected specimen were cut at 1–2 places according to different anatomical positions. If the skin lesion was located at the same anatomical site, four specimens were cut at the standard12, 3, 6 and 9 o’clock positions. Each specimen was cut from the visible skin lesion to the surgical resection margin. The width of the specimen was about 0.5cm
Evaluation under microscope
Photography was carried out with a scanning camera [Figures 2a and b]. The histopathologic tumor margin was adopted as the gold standard to determine the tumor margins. The observation included the following: (1) the distance between the macroscopic line and the histopathologic tumor margin; (2) the distance between the photodynamic diagnostic marker line and the histopathologic tumor margin; (3) the distance between the marker line of photodynamic diagnosis plus reflectance confocal microscopy and the histopathologic tumor margin; (4) if the tumor margin exceeded the marker line, it was considered to be false negative. If it did not exceed the marker line, it was considered to be negative [Figure 3].
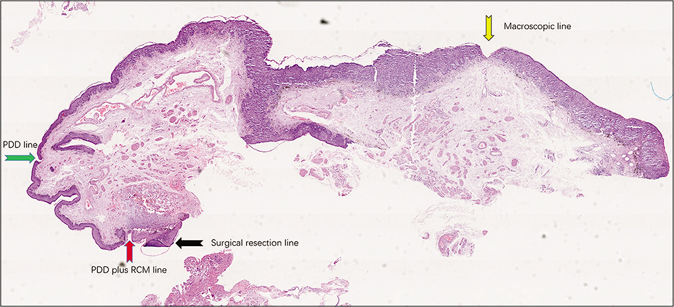
- Histopathology with different marker lines and tumor margin (H and E, ×40). The color arrows legends are the same as Figure 1d.The red box represents Figure 2b
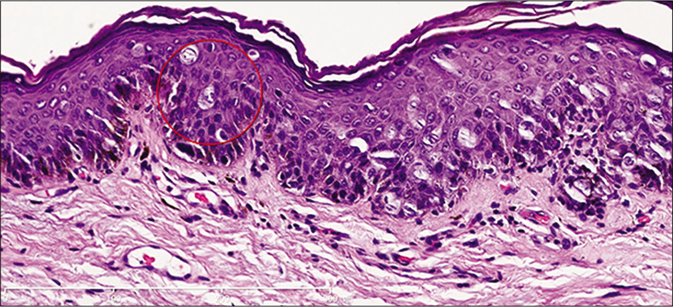
- Histopathology marking the margin of the tumor. The red circle-the margin of the tumor (H andE, ×200)
- Route map for detecting margins of extramammary Paget disease
Statistical methods
The number of cases diagnosed by the three methods was expressed as a frequency while the positive, sensitivity and false-negative rate were expressed as proportions. The Chi-square test was used to test the statistical differences between the sensitivity and false-negative rates of the three methods. A P value of < 0.05 was considered statistically significant.
Results
The patient cohort included 32 men and 4 women (age range, 50–82 years old). One man had axillary lesions, 31 had genital lesions and the 4 females had vulvar lesions. The disease course ranged from 4 months to 20 years. The area of the macroscopic skin lesions ranged from 0.5 cm × 1.0 cm to 11.4 cm × 15.7cm.
In total, 130 good quality tissue sections were selected from 36 patients and the tumor margin was observed under the microscope. The false-negative sections and sensitivities of the three methods were calculated and compared as follows [Table 1].
| Diagnostic lines | Scrotum (n=47), n(%) | Penis (n=26), n(%) | Pubic mound (n=38), n(%) | Groin (n=7), n(%) | Armpit (n=3), n(%) | Lower abdomen (n=3), n(%) | Perineum (n=6), n(%) | Total (n=130), n(%) |
|---|---|---|---|---|---|---|---|---|
| Macroscopy | 29 (61.7) | 17 (65.4) | 25 (65.8) | 5 (71.4) | 2 (66.7) | 2 (66.7) | 3 (50.0) | 83 (63.8) |
| PDD | 17 (36.2) | 6 (23.1) | 18 (47.4) | 3 (42.9) | 0 (0 | 1 (33.3) | 1 (16.7) | 46 (35.4) |
| PDD plus RCM | 10 (21.3) | 1 (3.8) | 12 (31.6) | 2(28.6) | 0 (0) | 1 (33.3) | 1 (16.7) | 27 (20.8) |
PDD: photodynamic diagnosis; RCM: reflectance confocal microscopy.
Relationship between the histopathologic tumor margins and the margins of the clinical macroscopic skin lesions: There were 83 sections outside the macroscopic line (false-negative rate 63.8% (83/130); mean distance outside the line, 3.5 ± 3.1mm; median, 2.7mm; maximum distance, 14.6mm). Among the 83 sections, 29 sections were in the scrotum, 17 sections were located in the penis and 25 sections were located in the pubic mound, and other 12 sections were located in groin, armpit, lower abdomen and perineum.
Relationship between the histopathologic tumor margins and the photodynamic diagnosis line: There were 46 sections located outside the photodynamic diagnostic marker line (false-negative rate 35.4% (34/130); mean distance, 2.1 ± 1.7mm; maximum distance, 5.9mm). Of the 46 sections located outside the marked line, 17 sections were from the scrotum, 6 sections were taken from the penis and 18 sections were located in the pubic mound, and other 5 sections were located in the other anatomical sites.
Relationship between the histopathologic tumor margins and the photodynamic diagnosis plus reflectance confocal microscopy line: The histopathologic tumor margins of 27 sections were located outside the photodynamic diagnosis plus reflectance confocal microscopy line (false negative 20.8% (27/130); mean distance outside the line, 1.4 ± 1.2mm; maximum distance, 5.3mm).
Relationship between the histopathologic tumor margins and the surgical margins: In 130 sections, the outer margin of the tumor was entirely located within the cutting edge. The distance from the outer margin of the tumor to the cutting edge was 8.9 ± 5.4mm with a minimum of 0.3mm and a maximum of 30.2mm.
Distance between the photodynamic diagnosis plus reflectance confocal microscopy mark line and the surgically cut edge: The average distance was 6.2 ± 3.3mm and the maximum distance was 23.0mm.
Among the 130 sections, 34 sections had one marker line and 30 sections had three marker lines. There were two marker lines in 66 sections, of which 35 were overlapped by the gross line and the photodynamic diagnostic line and 31 were overlapped by the photodynamic diagnostic line and photodynamic diagnosis plus reflectance confocal microscopy line.
All the visual observation lines and photodynamic diagnostic lines were either located inside of or overlapping, the photodynamic diagnosis plus reflectance confocal microscopy line. All macroscopic lines were either located inside of or overlapping, the photodynamic diagnostic line.
Table 1 summarizes the comparison of the 3 diagnostic lines as follows: Chi-square test was used for the sensitivity comparison among the three groups. There were statistically significant differences among the three groups (c2=51.99, P<0.0001). There were statistically significant differences between macroscopy and photodynamic diagnosis (c2=20.98, P<0.0001). There were statistically significant differences between macroscopy and photodynamic diagnosis plus reflectance confocal microscopy (c2=49.23, P<0.0001). There were statistically significant differences between photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy (c2=6.85, P=0.0089). Chi-square test was used for the sensitivity comparison among the three groups at scrotum site. There were statistically significant differences among the three groups (c2=16.41, P=0.0003). There were statistically significant differences between macroscopy and photodynamic diagnosis(c2=6.07, P=0.0138). There were statistically significant differences between macroscopy and photodynamic diagnosis plus reflectance confocal microscopy (c2=15.65, P=0.0001). There were no statistically significant differences between photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy (c2=2.52, P=0.11)
Discussion
Accurate and effective margin determination is key to good surgical outcomes and for some extrammary Paget disease lesions, unclear margins may be one of the causes of postoperative recurrence. The current study aimed to assess diagnostic methods that were noninvasive, convenient and quick.
The main principle of photodynamic fluorescence is that the photosensitizer is absorbed by the target cells and metabolized to porphyrin which displays specific fluorescence under a specific wavelength of light. The fluorescence can then show a clear margin line between the diseased and normal skin. This helps to determine the scope of extramammary Paget disease lesions and their margins and is considered a breakthrough in the diagnosis and treatment of tumors.5 Wang et al. applied 20% aminolaevulinic acid gel to the macroscopic skin lesions and 5 cm beyond the margins in patients with extramammary Paget disease and protected them from light for 4 h. The clinical and subclinical skin lesions showed brick-red fluorescence under ultraviolet light at wavelengths of 320–400 nm.6 Huang et al. found a subclinical lesion of extramammary Paget disease with a distance of 3cm from the principal lesion using a combination of photodynamic diagnosis and reflectance confocal microscopy.4
The Paget cells in extramammary Paget disease present as atypical cells inside the epidermis with highly refractive large cell nuclei under reflectance confocal microscopy. Busam et al. reported that confocal scanning laser microscopy could help operators to quickly identify the margins of skin malignancy lesions that need to be resected, assess the surgical scope and guide surgical resection.7 Tannous et al. pointed out that Mohs surgery relies on confocal scanning laser microscopy to guide the excision of skin tumors, maximizing the sparing of normal tissues and increasing the cure rate.8 Guitera et al. collected reflectance confocal microscopy images from nine extramammary Paget disease patients and one mammary Paget disease patient from three centers, as well as histopathological images from nine of these patients. By comparing the reflectance confocal microscopy images and histopathological findings, the authors suggested that reflectance confocal microscopy is not only important in the diagnosis of extramammary Paget disease but can also be used to mark the tumor margins before surgery.9 Pan et al. found that the lesion characteristics observed in extramammary Paget disease by reflectance confocal microscopy correspond well to the pathological findings and that reflectance confocal microscopy can be used as an adjunct to the diagnosis and management of extramammary Paget disease.10
However, due to the structure of the reflectance confocal microscopy instruments and the small visual field, it takes around 3–5 min to detect each visual field and multiple continuous observations are needed to obtain the microscopy marker line. If the skin lesion area is large, the process may take some time, although there are available handheld reflectance confocal microscopy devices that can overcome the limitations of the reflectance confocal microscopy robotic arm.11 Furthermore, it is challenging to perform large-area examinations on uneven skin, such as the scrotum which has many skin wrinkles and strong skin extensibility. Considering these shortcomings, we simplified the detection procedure by first labeling the tumor margin visible to the naked eye, then performing the photodynamic fluorescence diagnosis by extending 5cm outward from the macroscopic line and marking the photodynamic diagnostic marker line. On this basis, reflectance confocal microscopy was employed to continue to detect the margin of the tumor along the photodynamic diagnostic marker line. If tumor cells were observed, expansion was continued outward until no further tumor cells were seen. This has the potential to simplify the reflectance confocal microscopy detection process and minimize the time the process takes.
The areas outside the marked lines represent the negative results of each examination method. However, a pathological examination of the area showed that tumor cells were still present in some samples, so we took statistically positive data and tested the sensitivity. This found that the positive rate, that is, the sensitivity of the clinical visual observation, photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy detection were 36.2%, 64.6% and 79.2%, respectively. The percentage of false negatives by photodynamic diagnosis plus reflectance confocal microscopy detection was lower than that of photodynamic diagnosis alone and clinical visual observation. Photodynamic diagnosis alone demonstrated a lower proportion of false negatives than visual observation. The study demonstrated that photodynamic diagnosis plus reflectance confocal microscopy and photodynamic diagnosis alone are superior to visual observation for establishing the tumor margin while photodynamic diagnosis plus reflectance confocal microscopy may be more efficacious in improving the diagnostic rate of assessing tumors margins.
This study also shows that in the scrotum, penis and pubic area, the proportion of false negatives of the tumor margin exceeding the macroscopic line, photodynamic diagnosis, photodynamic diagnosis plus reflectance confocal microscopy detection line are higher than other parts. Of these, the scrotum has the highest proportion of false negatives. This may be related to the anatomical characteristics of the scrotum, including the high number of folds and skin extensibility. Due to the small number of cases, the proportion of positive values in some parts, such as the armpit, lower abdomen, groin and perineum, are relatively high. Therefore, further study of these parts is required.
Limitations
Our study has several limitations. Photodynamic diagnosis, reflectance confocal microscopy and naked eye observation can only observe the surface margin of the tumor and not deeper areas. Besides, reflectance confocal microscopy was not used alone as a control.
Conclusion
In conclusion, this study examined the distance between the histopathologic tumor margin and the macroscopic line, photodynamic diagnosis line and the photodynamic diagnosis plus reflectance confocal microscopy line in histopathological sections. Photodynamic diagnosis and photodynamic diagnosis plus reflectance confocal microscopy are superior to visual observation in the noninvasive determination of the extramammary Paget disease margins. These results will prove useful for assessing tumor margins and surgical scope and help to improve the treatment of this disease.
Acknowledgment
Thanks to Professor Xiangdong Gong of the Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College for his guidance in statistics of this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
(1) Scientific Research Project of Jiangsu Commission of Health (BJ16007); (2) Youth Medical Key Talents with National Tutorial System Training Program of Suzhou (GGRC028).
Conflicts of interest
There are no conflicts of interest.
References
- Comparison of Mohs micrographic surgery and wide excision for extramammary Paget's disease: Korean experience. Dermatol Surg. 2009;35:34-40.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of extramammary Paget disease of the vulva with imiquimod: A retrospective, multicenter study by the German Colposcopy Network. J Am Acad Dermatol. 2014;70:644-50.
- [CrossRef] [PubMed] [Google Scholar]
- Extramammary Paget disease: Surgical treatment with Mohs micrographic surgery. J Am AcadDermatol. 2004;51:767-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of subclinical extramammary Paget's disease with a combination of noninvasive photodynamic diagnosis and reflectance confocal microscopy. Indian J Dermatol Venereol Leprol 2019
- [CrossRef] [Google Scholar]
- Fluorescence diagnostics as a guide for demarcation and biopsy of suspected anal cancer. Int J Dermatol. 2012;51:31-4.
- [CrossRef] [PubMed] [Google Scholar]
- Complete remission of two patients with recurrent and wide spread extramammary Paget disease obtained from 5 aminolevulinic acid based photodynamic therapy and imiquimod combination treatment. Photodiagnosis Photodyn Ther2014;. ;11:434-40.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-9.
- [Google Scholar]
- In vivo real time confocal reflectance microscopy: A noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol Surg2003;. ;29:839-46.
- [CrossRef] [PubMed] [Google Scholar]
- Reflectance confocal microscopy for diagnosis of mammary and extramammary Paget's disease. J Eur Acad Dermatol Venereol. 2013;27:e24-9.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo reflectance confocal microscopy of extramammary Paget disease: Diagnostic evaluation and surgical management. J Am Acad Dermatol. 2012;66:e47-53.
- [CrossRef] [PubMed] [Google Scholar]
- Handheld Reflectance Confocal Microscopy for the Detection of Recurrent Extramammary Paget Disease. JAMA Dermatol2017;. ;153:689-93.
- [CrossRef] [PubMed] [Google Scholar]






