Translate this page into:
Utility of serum ERAP1 and ERAP2 as novel biomarkers in the management of recalcitrant warts: A cross-sectional study
Corresponding author: Dr. Karem Taha Khalil, Department of Dermatology, Venereology and Andrology, Benha University Faculty of Medicine, Benha, Egypt. karem.khalil@fmed.bu.edu.eg
-
Received: ,
Accepted: ,
How to cite this article: Younis I, Fouad NA, Ibrahim OS, Khalil KT. Utility of serum ERAP1 and ERAP2 as novel biomarkers in the management of recalcitrant warts: A cross-sectional study. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1541_2024
Abstract
Background
The exact mechanisms underlying the eradication of the human papillomavirus (HPV) by cellular immunity remain obscure. Individuals with treatment-resistant warts frequently have immune deficiencies. Amino-peptidases (ERAP1, ERAP2) of the endoplasmic reticulum are essential for the production of antigenic epitopes that attach to the Major Histocompatibility Complex (MHC) class I and activate T-lymphocytes or natural killer (NK) cells.
Aim
To assess ERAP1 and ERAP2 serum concentrations in patients with resistant warts.
Methods
The current study included 200 subjects. They were split into 2 groups. Group (I) patients had resistant warts (n = 100), and group (II) age- and sex-matched subjects had a history of treated warts with no recurrence (n = 100). Clinical assessment and ERAP1 and ERAP2 serum level determination via ELISA were conducted.
Results
ERAP1 and ERAP2 levels were significantly lower in group (I) than in group (II) (p < 0.0001 for each). A significant positive relationship was observed between ERAP1 and ERAP2 (p < 0.001). A significant negative correlation was found between ERAP1, ERAP2, the number of warts, and a number of recurrences (p < 0.001).
Limitations
The small sample size, the lack of measurements of ERAP1 and ERAP2 before and after the treatment, and the exclusion of medical conditions such as diabetes mellitus and hypertension.
Conclusion
The current study findings highlight the potential utility of serum ERAP1 and ERAP2 as novel biomarkers for identifying patients with recalcitrant warts.
Keywords
Amino-peptidase
ERAP1
ERAP2
warts
Introduction
Warts are benign epidermal cell proliferations generated by human papillomaviruses (HPVs), which are DNA viruses with a particular affinity for keratinocytes.1 About 5% of human malignancies are caused by persistent infections with oncogenic HPVs.2
Although most warts disappear on their own in months or years, there are instances of resistant warts continuing to grow despite numerous treatment attempts. Although the precise process is unknown, cellular immunity is essential for the clearance of HPV infections. Individuals with large, enduring, or treatment-resistant warts may have immune system deficiencies that contribute to their vulnerability to HPV.3
One-third of non-genital warts are resistant to immunotherapy and the majority of conventional therapies. It seems that the prevalence of resistance is rising due to the increasing immunosuppressant use.4
Antigens on MHC are monitored by the immune system in order to distinguish the infections. Endoplasmic reticulum amino-peptidases (ERAP1 and ERAP2) trim antigenic peptides broken down in proteasomes to the proper lengths for presentation on MHC-I molecules and subsequent activation of T-cells or NK-cells. Accordingly, ERAP1 and ERAP2 may be targets for viral infections and autoimmune disorders.5
This study aimed to assess serum concentrations of ERAP1 and ERAP2 in patients with recalcitrant warts.
Methods
Study design and population
A cross-sectional design was applied. Patients (200) were divided into two groups. Group (I) patients had resistant warts (n=100), and group (II) age- and sex-matched subjects with history of treated warts of matched sites and types with no recurrence (n=100). They were recruited from the Dermatology Outpatient Clinic at Benha University Hospitals between October 2022 and March 2024.
The literature does not provide a clear definition of recalcitrant warts. Refractory warts were classified as such if they had been treated with more than two standard techniques and were still unresponsive or if they had persisted for more than two years.6 Patients in Group I had a history of incomplete clearance or recurrence following two or more electrocautery sessions, four or more cryocautery treatments, and intralesional bleomycin.
However, patients with genital warts, those on systemic steroids or other immunosuppressive treatments, immunocompromised individuals, those suffering from chronic systemic diseases, and pregnant women were excluded.
Ethics
The protocol of work was approved by the Benha Faculty of Medicine’s Ethics Committee on Human Subjects Research, Egypt (MS.43.9.2022) and registered with ClinicalTrials.gov (number NCT06833164). Prior to participation, all individuals provided informed consent.
Data collection and Laboratory investigations
Each participant gave a full medical history, focusing on the duration of warts, previous treatments, and a number of recurrences. Cutaneous examination was done to determine the type, site, number, and sizes of warts. Commercial human ELISA kits were used to measure serum levels of ERAP1 (Catalogue number: SRB-T-83718, Sensitivity:0.045ng//mL, Range: 0.05-15ng/mL) and ERAP2 (Catalogue number: 201-12-8104, Sensitivity:10.25ng//L, Range: 15-3500ng/L), Shanghai SunRed Biological Technology Co., Ltd., China.
Statistical analysis
The data was evaluated by SPSS version 28 (IBM, Armonk, NY, USA). The normality of quantitative data was determined using the Shapiro-Wilk test. Means and standard deviations were used to represent quantitative data, whereas numbers and percentages were used for categorical data. Comparison between the groups was done with student’s t-test, Mann-Whitney U test, Chi-squared test, or Kruskal-Wallis test. Receiver operating characteristic (ROC) curves were used to determine the best cutoff point for each marker. The Pearson correlation coefficient was applied to show correlations between different variables. A P-value<0.05 was considered significant.
Results
The mean age of group (I) was 25.52 ± 10.65 years and group (II) was 27.91 ± 7.77 years, with no significant difference (p = 0.07). Both groups were sex matched (p = 0.4). The ERAP1 level was significantly lower in group (I) (2.21 ± 1.22 ng/mL) than in group (II) (11.1 ± 4.1 ng/mL) (p < 0.001). The ERAP2 level in group (I) (1.21 ± 0.7ng/mL) was significantly lower than in group (II) (6.2 ± 2.5ng/mL) (p < 0.001) [Table 1, Figure 1].
| Group (I) (n=100) | Group (II) (n=100) | Test of significance | p-value | |
|---|---|---|---|---|
|
Age (year) mean ± SD |
25.52±10.65 | 27.91±7.77 | t =1.8 | 0.07 |
| Sex n (%) | ||||
| Female | 50 (50) | 60(60) | X2=2.02 | 0.15 |
| Male | 50 (50) | 40(40) | ||
|
ERAP1 (ng/ml) mean ± SD |
2.21 ± 1.22 | 11.1 ± 4.1 | t =20.8 | <0.0001* |
|
ERAP2 (ng/ml) mean ± SD |
1.21 ± 0.7 | 6.2 ± 2.5 | t =19.2 | <0.0001* |
t: Student t-test, X2: Chi-square, SD: Standard deviation, * Significant p- value <0.05
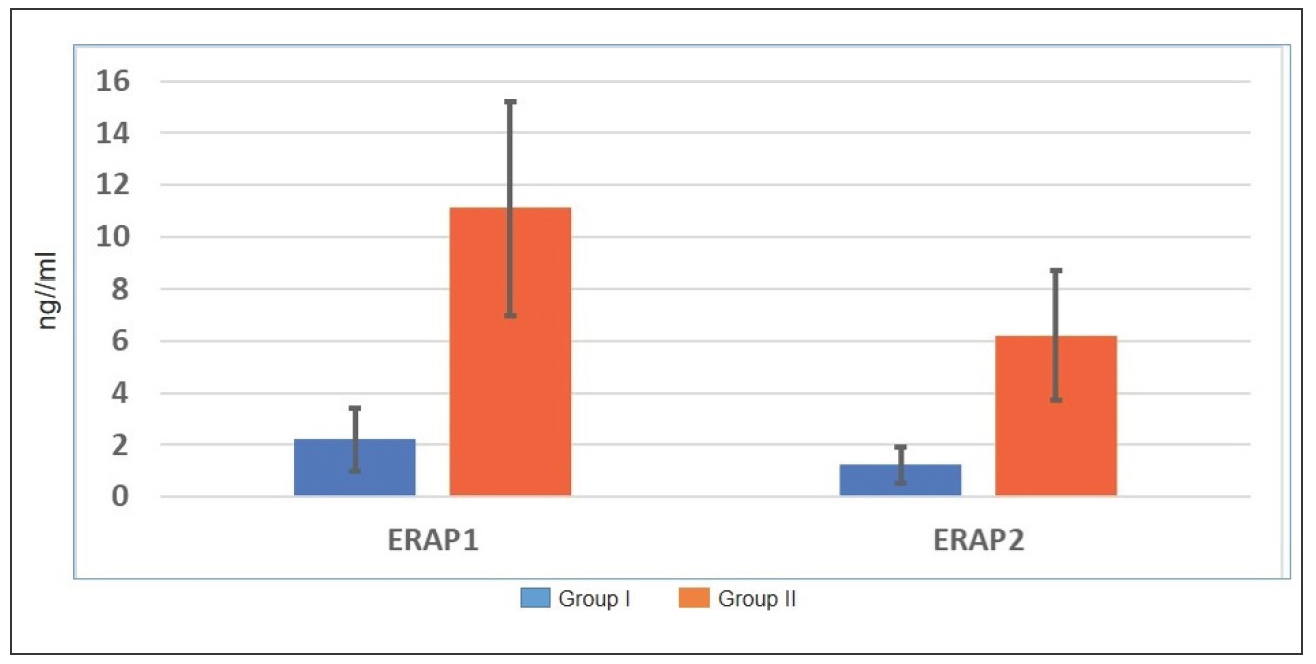
- Serum levels of ERAP1 and ERAP2 in the study groups.
ERAP1 and ERAP2 levels did not differ significantly for gender (p = 0.1, 0.3, respectively) and family history of recurrent warts (p = 0.5, 0.6, respectively) [Table 2].
| Variables | Male (n=50) | Female (n=50) | U | p-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| ERAP1 (ng/mL) | 1.96 ± 0.9 | 2.5 ± 1.5 | 1.5 | 0.1 |
| ERAP2 (ng/mL) | 1.1 ± 0.6 | 1.3 ± 0.9 | 1.1 | 0.3 |
| Positive family history of recurrent warts (n=52) | Negative family history of recurrent warts (n=48) | |||
| Mean ± SD | Mean ± SD | |||
| ERAP1 (ng/mL) | 2.3 ± 1.3 | 2.1 ± 1.1 | 0.4 | 0.5 |
| ERAP2 (ng/mL) | 1.2 ± 0.7 | 1.3 ± 0.8 | 0.7 | 0.6 |
U: Mann-Whitney U-test, SD: Standard deviation
There was a significant difference in ERAP1 levels (p = 0.04) according to the site of warts but not in ERAP2 (p = 0.4 ) [Table 3].
| Variables |
n. |
Mean ± SD | K-W | p-value | |
|---|---|---|---|---|---|
| ERAP1 (ng/mL) | Periungual warts | 10 | 3.6 ± 1.4 | 9.5 | 0.04* |
| Plantar warts | 26 | 1.9 ± 0.7 | |||
| Palmar warts | 16 | 1.9 ± 0.8 | |||
| Plane warts | 20 | 2.9 ± 1.7 | |||
| Common warts | 28 | 1.6 ± 0.7 | |||
| ERAP2 (ng/mL) | Periungual warts | 10 | 1.7 ± 0.9 | 4.4 | 0.4 |
| Plantar warts | 26 | 1.1 ± 0.5 | |||
| Palmar warts | 16 | 1.1 ± 0.6 | |||
| Plane warts | 20 | 1.6 ± 0.2 | |||
| Common warts | 28 | 0.9 ± 0.4 | |||
Kruskal-Wallis test=K-W, *Significant p-value<0.05
Significant positive correlation was detected between ERAP1 and ERAP2 (r = 0.8, p < 0.001). ERAP1 and 2 were negatively correlated with the number of warts and the number of recurrences with statistical significance (p <0.001 for both). However, ERAP1 and 2 had no significant correlation with other variables, including the age of the patient, duration of the disease, and size of warts (p > 0.05 for all) [Table 4, Figure 2].
| Variables | ERAP1 | ERAP2 | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Age | -0.04 | 0.8 | 0.1 | 0.5 |
| Duration of disease | 0.2 | 0.2 | 0.2 | 0.2 |
| Size of warts | 0.1 | 0.4 | -0.04 | 0.8 |
| Number of warts | -0.79 | <0.001* | -0.66 | <0.001* |
| Number of recurrences | -0.8 | <0.001* | -0.6 | <0.001* |
| Serum ERAP2 | 0.8 | <0.001* | ||
r: Pearson correlation coefficient, SD: Standard deviation, K-W: Kruskal-Wallis test, *Significant p-value<0.05
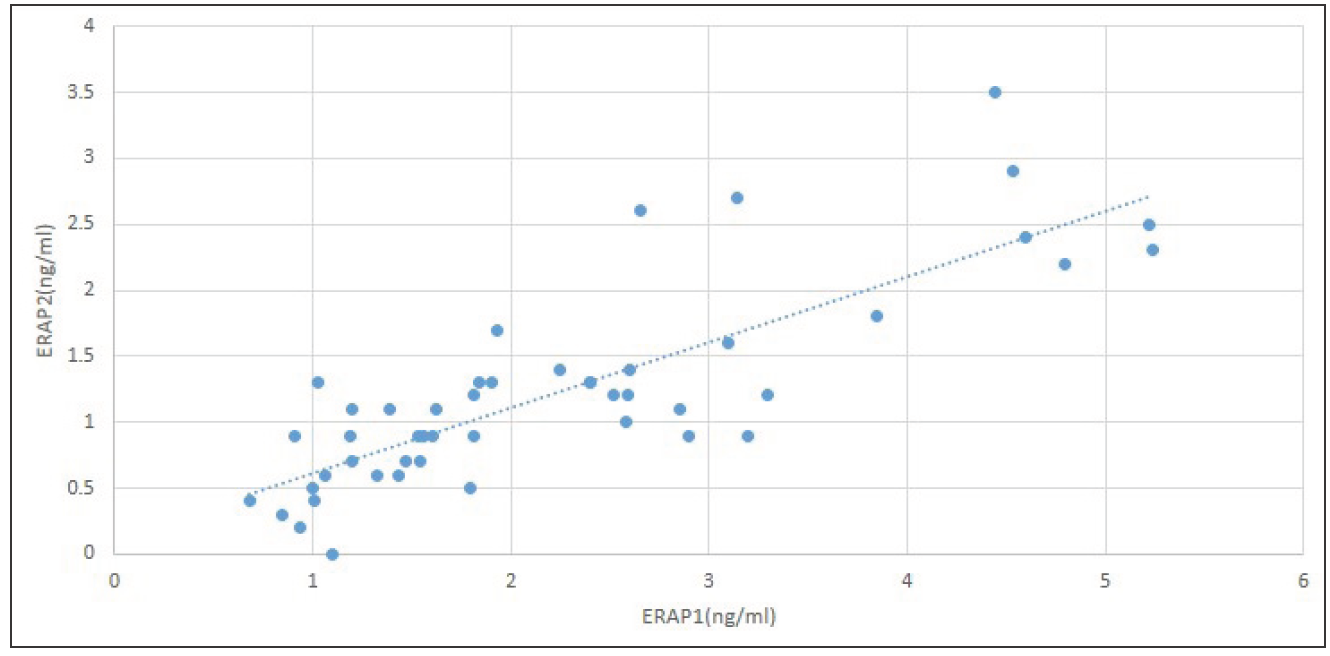
- Correlation between serum ERAP1 and ERAP2.
Optimal cutoff points of serum ERAP1 and ERAP2 levels significantly distinguish between patients with recalcitrant warts and those with non-recurrent warts where the area under the curve (AUC) equals 0.88 and 0.84, respectively, and a significant p-value <0.001 [Table 5, Figure 3].
| Variables | AUC | 95% CI | Cutoff | Sensitivity | Specificity | p-value |
|---|---|---|---|---|---|---|
| ERAP1 (ng/mL) | 0.88 | 0.8-0.97 | 4.50 | 88 | 90 | <0.001* |
| ERAP2 (ng/mL) | 0.84 | 0.75-0.93 | 2.35 | 81 | 86 | <0.001* |
ROC: Receiver operating characteristic, AUC: Area under curve, CI: Confidence interval, *Significant p-value <0.05
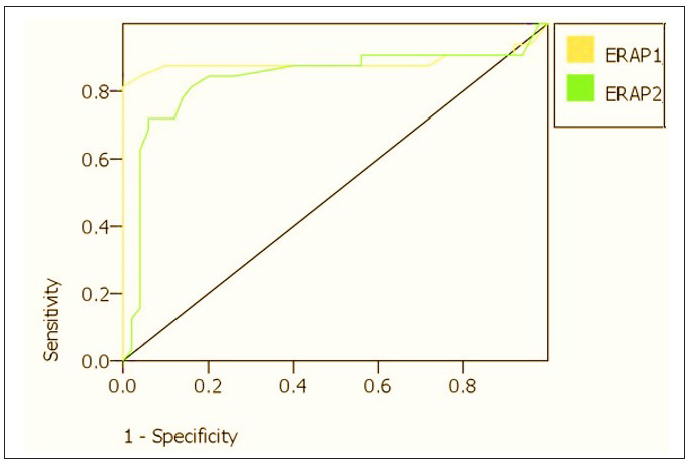
- ROC curves for serum ERAP1 and ERAP2 in the prediction of recalcitrant warts
Discussion
ERAPs can be released into the extracellular environment and undertake a number of pathogenic activities in addition to their role as antigen-processing enzymes.7-9 To our knowledge, this is the first study to assess serum levels of ERAP1 and ERAP2 in patients with recalcitrant warts. The present study found that patients with resistant warts had considerably lower ERAP1 and ERAP2 levels than those with no recurrence. Moreover, ERAP1 and ERAP2 showed an evident negative correlation with the number of warts and recurrence rate.
ERAP1, a zinc metallopeptidase, trims peptides and cleaves proinflammatory cytokine receptors like TNF-α, IL-6, IL-1 α, and IL-1 β to modulate signal intensity on the cell surface.10 These cytokines, produced by CD4+ and CD8+ T-cells, are linked to wart regression.11 Disruption or absence of ERAP function can change the antigens presented by MHC I molecules, influencing the activation of NK cells and CD8+ T cells. This can result in a weakened immune response and illness development.12
Previous studies have described the dysregulation of ERAP1 in many conditions, such as HIV, lymphocytic choriomeningitis virus (LCMV), human cytomegalovirus (HCMV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infection.13-17
The results of this research showed a strong positive association between ERAP1 and ERAP2. The previously published studies demonstrated that ERAP1 and ERAP2 work together to produce complete epitopes that bind to MHC I. It was discovered that ERAP2 physically associates with ERAP1 and exhibits unique specificity for the N-terminal of the peptides. It is anticipated that this complex handles the many precursor peptides more effectively than individual enzymes.18
In line with the positive correlation between ERAP1 and ERAP2, Kemming et al. found that variation in one ERAP-coding gene influences the function of the other, modifying the immune reaction to HCV.19
However, ERAP’s role may be context dependent. In mice with HLA-B27, the deletion of ERAP resulted in a substantial reduction in CD8+ T response to influenza epitopes. In contrast, ERAP was not required for peptide processing in the presence of HLA-B7.20
Limitations
Limitations of this study include the small sample size, the lack of measurements of ERAP1 and ERAP2 before and after treatment, and the exclusion of medical conditions such as diabetes mellitus and hypertension. Further prospective studies with analysis of ERAP1 and ERAP2 coding gene polymorphisms are necessary to validate the current findings and elucidate the underlying mechanisms. Correlations between tissue expression and serum levels of ERAP1 and ERAP2 will help clarify their role in the pathogenesis of warts. The correlation of ERAPs with HPV viral load and different HPV serotypes needs to be assessed in future studies.
Conclusion
ERAP1 and ERAP2 play critical roles in the immunity against HPV infections. Dysregulation of these amino-peptidases may contribute to the persistence and aggression of warts. Incorporating such markers into clinical practice may aid in early detection, risk stratification, and individualised treatment options for patients with resistant warts.
Ethical approval
The research/study was approved by the Institutional Review Board at Benha Faculty of Medicine’s Ethics Committee on Human Subjects Research, number MS.43.9.2022, dated 25-2-2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- HPV type‐specific distribution among family members and linen in households of cutaneous wart patients. Acad Dermatol Venereol. 2022;36:119-25.
- [Google Scholar]
- Human papillomavirus infection in solid organ transplant recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13590.
- [CrossRef] [PubMed] [Google Scholar]
- Consideration of underlying immunodeficiency in refractory or recalcitrant warts: A review of the literature. Skin Health Dis. 2022;2:e98.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Recalcitrant plantar warts during azathioprine therapy for Crohn’s disease. Ann Gastroenterol. 2013;26:173-4.
- [PubMed] [PubMed Central] [Google Scholar]
- Functional ERAP1 variants distinctively associate with ankylosing spondylitis susceptibility under the influence of HLA-B27 in taiwanese. Cells. 2022;11:2427.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical guideline for the diagnosis and treatment of cutaneous warts. J Evid Based Med. 2022;15:284-301.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoplasmic reticulum aminopeptidase 1 beyond antigenic peptide-processing enzyme in the endoplasmic reticulum. Biol Pharm Bull. 2020;43:207-14.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated analysis of fine-needle-aspiration cystic fluid proteome, cancer cell secretome, and public transcriptome datasets for papillary thyroid cancer biomarker discovery. Oncotarget. 2018;9:12079-100.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoplasmic reticulum associated aminopeptidase 2 (ERAP2) is released in the secretome of activated MDMs and reduces in vitro HIV-1 infection. Front Immunol. 2019;10:1648.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of ERAP1 gene single nucleotide polymorphism in impressing the inflammatory cytokine profile of ankylosing spondylitis patients. Iran J Allergy Asthma Immunol. 2018;17:464-7.
- [CrossRef] [PubMed] [Google Scholar]
- Warts and all: Human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-48.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An overview on ERAP roles in infectious diseases. Cells. 2020;9:720.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol. 2009;10:636-46.
- [CrossRef] [PubMed] [Google Scholar]
- Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class i-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Human cytomegalovirus microRNA miR-US4-1 inhibits CD8+ T cell responses by targeting the aminopeptidase ERAP1. Nat Immunol. 2011;12:984-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoplasmic reticulum aminopeptidase 1 is involved in anti-viral immune response of Hepatitis B virus by trimming Hepatitis B core antigen to generate 9-mers peptides. Front Microbiol. 2022;13:829241.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoplasmic reticulum aminopeptidases: Biochemistry, physiology and pathology. J Biochem. 2013;154:219-28.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between ERAP alleles and HLA Class I types support a role of antigen presentation in Hodgkin lymphoma development. Cancers (Basel). 2021;13:414.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ERAP1 allotypes shape the epitope repertoire of virus-specific CD8+ T cell responses in acute hepatitis C virus infection. J Hepatol. 2019;70:1072-81.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol. 2012;143:99-115.
- [CrossRef] [PubMed] [Google Scholar]







