Translate this page into:
Utility of trichoscopy to diagnose early female pattern hair loss in resource-poor setting: A cross-sectional study
2 Department of Dermatology, Venereology and Leprosy, GMERS Medical College, Civil Hospital, Junagadh, Gujarat, India
Correspondence Address:
Rahul Nagar
Department of Dermatology, Venereology and Leprosy, II Floor, New OPD Complex, MY Hospital Campus, Indore - 452 001, Madhya Pradesh
India
| How to cite this article: Nagar R, Dhudshia R. Utility of trichoscopy to diagnose early female pattern hair loss in resource-poor setting: A cross-sectional study. Indian J Dermatol Venereol Leprol 2019;85:681 |
Abstract
Background: Trichoscopy is a reliable instrument for diagnosis and for tracking therapy-related changes in female pattern hair loss (FPHL). Videodermoscopic diagnosis of FPHL has been established, which requires fine measurements of hair-related parameters; the method requires an expensive equipment/digital program.
Aim: To determine whether a low-cost, simple USB dermoscope can ascertain the hair-related changes in early FPHL.
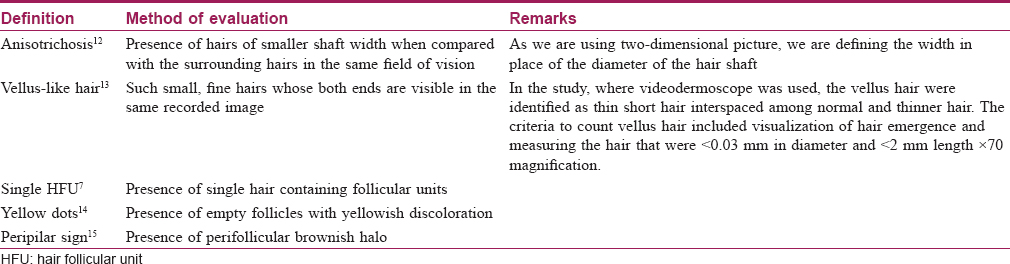
Methods: An age-matched, cross-sectional study was performed over 3 years on subjects with less than 6-month history of hair loss and without an obvious broadening of midline hair parting. Trichoscopic analysis of the frontal and occipital scalp of the study subjects were performed, using a USB-connected dermoscope. The subjects were analyzed for the presence of microscopic hair changes in the form of anisotrichosis, vellus-like hair, single hair follicle unit, peri-pilar sign and yellow dots.
Results: A total of 230 cases and 230 controls were analyzed. The dermoscopic hair changes were found to be significantly associated with the frontal scalp zone of cases.
Limitations: Histopathological evaluation of the cases was not done.
Conclusion: Microscopic changes recorded with the help of a simple USB dermoscope are helpful in establishing a diagnosis of FPHL even in early disease.
Introduction
Female pattern hair loss (FPHL) is a non-scarring progressive thinning of hair, with follicular miniaturization in a patterned distribution. Various clinical patterns of hair loss have been defined and used in the scales for assessing FPHL. Diffuse visible thinning of the crown region with preservation of frontal hairline have been described in Ludwig scale and Sinclair scale.[1],[2] Similarly, thinning and widening of the central part of the scalp with the breach of frontal hairline have been described in Olsen Christmas tree pattern.[3] Also, thinning associated with the bitemporal recession is defined in Hamilton–Norwood scale.[4] Scales like Ludwig's require assessing the visible hair thinning on the crown, which is the reason why it fails to detect the early-stage FPHL.[5] The midline part and Christmas tree pattern are undoubtedly valuable clinical criteria in assessing FPHL, but they reflect the advanced stages of the condition. Thus, these scales are less useful for the diagnosis of an early stage of FPHL.[5]
Trichoscopy helps in better visualization of hair and scalp thereby aiding in the diagnosis of FPHL. The disease is characterized by the increased diversity of hair diameters, increased number of thin hairs (vellus-like hair), and an increased number of single hair containing follicular units, yellow dots, and peripilar sign.[6]
Recently, videodermoscopy criteria have also been published by Rakowska et al.[7] These are as follows: Major – (1) >4 yellow dots in four images in frontal area, (2) lower average hair thickness in the frontal area compared with occiput area, and (3) more than 10% of thin hairs (<0.03 mm) in the frontal area.
Minor – (1) ratio of single hair unit percentage, frontal to occiput > 2:1, (2) number of vellus hair, >1.5:1, and (3) ratio of perifollicular discoloration, >3:1.
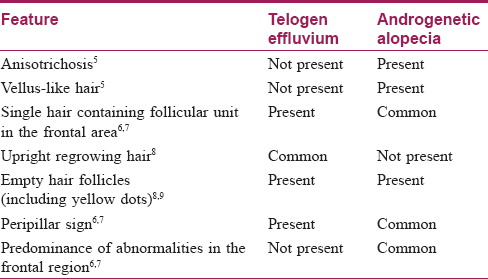
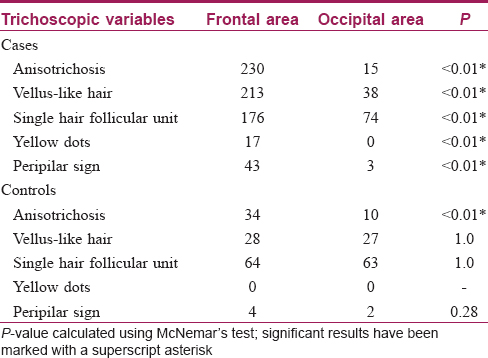
The result of this study also indicates that FPHL may be differentiated from chronic telogen effluvium [Table - 1].
The use of specialized instruments and dedicated software in this study allows for the precise measurement of hair-related structural changes. However, owing to high cost, these high-end videodermoscopes are not readily available to most of the dermatologists. Besides, this study does not demarcate the trichoscopic changes according to either the duration or severity of the disease.
Identifying the changes early in FPHL are more relevant, as an appropriate therapeutic intervention at this stage will halt the progression of FPHL.[5] If we could quantify the dermoscopic changes associated with early stages of FPHL, this could prove of great help, even greater if we could assess it using inexpensive dermoscopes. Trichoscopy may be performed with handheld dermoscopes, or with basic digital dermoscopes and photographic equipment, or with advanced digital dermoscopes.[6] Handheld dermoscopes may be divided into three groups: contact dermoscopes, polarized light contact dermoscopes, and polarized light noncontact dermoscopes.[6] Also available are handheld dermoscopes that work in either the contact or noncontact mode; these are known as hybrid dermoscopes. Which device to choose is a matter of individual preference; there is no preferred type of dermoscope for performing hair and scalp examinations. The standard magnification of handheld dermoscopes is ×10; the cost varies between about US $700 and US $1,800[6](In India can get between 1000 rupees to 10,000 rupees). New devices on the market include simplified digital dermoscopes that may be connected to a computer (e.g., via USB) and kits allowing one to connect selected handheld dermoscopes to a regular photo camera. The usual magnification is ×10 to ×80, depending on the device.[6] The price of these devices varies between US $400 and US $2,000 (not including the computer, camera, or phone) The large, expensive digital dermoscopes (videodermoscopes) allow one to take high-magni fi cation, high-quality photographs.[6] The price of these devices varies signi fi cantly, depending on the presence or absence of software. This type of digital dermoscope offers multiple magni fi cations in the range of ×20 to ×70 (or ×100) and higher. The price varies from about US $10,000 to about US $20,000.[6] Low-cost versions, unlike high-end videodermoscopes, are unable to take exact measurements of FPHL-related variables. However, it may record a fairly good quality picture and it may be able to produce digital images capable of storage. Hence, we wished to assess whether a simple Universal Serial Bus (USB)-cable-connected dermoscope could be useful to aid in the diagnosis of early-stage FPHL. A dermoscope of this kind has to be connected to the computer through a USB port. Afterwards, the image through the dermoscope could be captured and recorded using image-capturing software. Some of the image-capturing software are available free of charge over World Wide Web (see below).
Follicular miniaturization in the scalp is the hallmark of FPHL.[8] There is some role of androgens and genetic susceptibility described in this transformation. The higher level of 5α reductase and androgen receptors in frontal hair follicles probably explains patterned hair loss sparing the occiput. Increased aromatase enzyme activity in females also protects the scalp from undergoing total baldness.[8] Thus, logically we may assume that early changes in FPHL would be more pronounced in the frontal scalp, rather than any other part. Also, the occipital part would not be affected in patterned hair loss.
Therefore, we planned to evaluate and compare frontal and occipital scalps of study subjects. The evaluation was performed using an inexpensive USB dermoscope. We wanted to establish criteria associated with early FPHL using such a device. This would help in the diagnosis of future patients of early FPHL with the convenience of rapid, low-cost, and easy-to-use USB dermoscope.
Methods
This was a cross-sectional study performed at the outpatient facility in the Department of Dermatology, Venereology and Leprosy of Mahatma Gandhi Memorial Medical College, Indore, India. During this study, trichoscopic images from the frontal and occipital scalp of females suffering from early patterned hair loss and healthy controls were analyzed. Females belonging to the age group of 18–35 years with the subjective complaint of hair loss of duration not more than 6 months were analyzed by USB dermoscope. We considered that 6 months history of hair loss would be sufficient to assume early FPHL. A longer duration of hair loss would produce a clinically diagnosable parting in midline scalp. We wanted to study early FPHL where clinically significant parting has not occurred. Thus, patients with the width of central parting not extending beyond 1 cm were included [Figure - 1]. Inclusion criterion of hair parting span of less than 1 cm was arbitrarily chosen. There are several reasons for accepting this criterion. First, current literature lacks any precise definition of midline hair parting spread to differentiate early from late patterned hair loss. Second, we think parting spread of more than 1 cm could be sufficient enough to make a clinical diagnosis of patterned hair loss obviating the need for a dermoscopy. Third, the objective of our study is to assess the utility of trichoscopy in evaluating early FPHL, where the patient has still not lost enough hair density for an obvious clinical diagnosis. Furthermore, analysis of central parting is important in FPHL as suggested by Hung et al.[9]
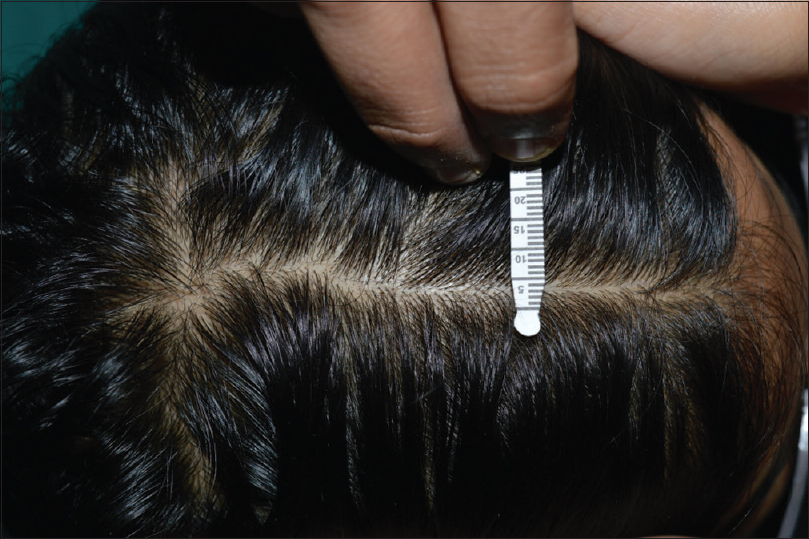 |
| Figure 1: Measurement of midline parting spread using a Schirmir's tear test strip; this case had different dermoscopic findings in her frontal scalp compared with the occipital scalp |
Because pattern hair loss predominantly affects frontal scalp, subjects with the relative dermoscopic difference in hair-related variables in frontal and occipital areas were only recruited as cases. Patients with hair loss with similar occipital and frontal dermoscopic findings may be suffering from telogen effluvium instead of FPHL,[7],[10] and thus were excluded from the study.
Patients with hair loss following an acute illness or following pregnancy were excluded. Females with other obvious hair and scalp disorders either primary like trichotillomania, alopecia areata, or tinea capitis or secondary like seborrheic dermatitis or psoriasis were also excluded. Patients who were on any type of anti-hair loss treatment or patients with endocrine disorders or patients on systemic allopathic or alternative medications were excluded.
Age-matched controls without history of hair loss were recruited from hospital patients and attendants. The controls were recruited during the same period of time as the cases. For each case, the control was recruited using the same exclusion criteria.
All the subjects were asked to shampoo their hair 1 day prior to the procedure. They were asked to avoid oiling or coloring their hair. A straight parting was obtained at the mid-scalp with a fine-toothed comb. Trichoscopy was performed using a USB-cable-connected dermoscope manufactured by Tejco Vision ® (Mumbai, India), which provides scalp visualization at 5 × to 200×. Snap ® software was used to analyze and record the dermoscopic images. Snap ® software is a free web-app available for download from the World Wide Web.
It has been suggested that in patterned hair loss, trichoscopic examination should be performed in the frontoparietal area approximately at the cross between the nose line and ear implantation.[11] Also, Rakowska et al. have suggested that the trichoscopic evaluation of temporal area may be ignored in dermatology practice.[7] Furthermore, pattern hair loss–induced dermoscopy changes observed in the frontal area are more pronounced compared to the occipital area. Considering the above facts, the frontal and occipital scalp were examined in the midline (sagittal plane) approximately 3 cm above the hairline at 50× magnification that covers the area of 5 × 7 mm [2]. All the images evaluated for the hair changes are presented in [Table - 2].
Frontal scalp and occipital scalp of cases were compared with that of controls. Similarly, frontal scalp of cases was compared with the occipital area of their own.
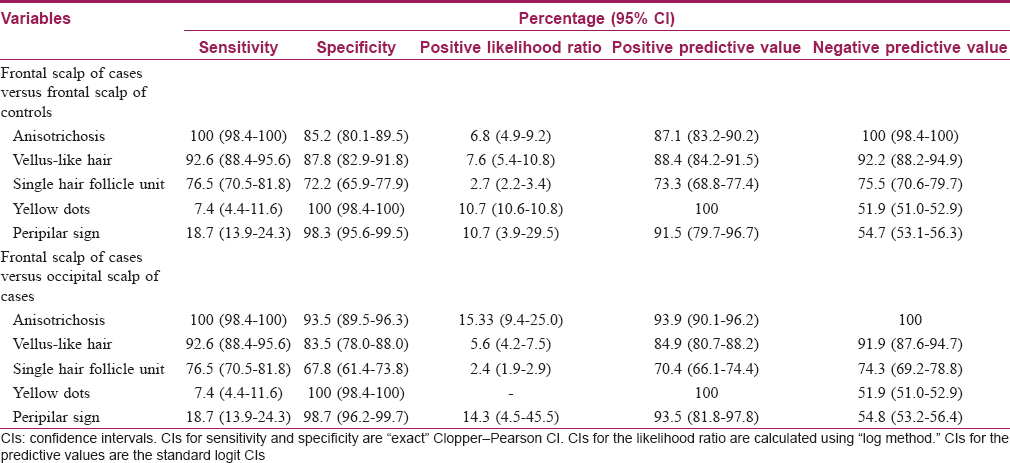
Appropriate statistical analysis was performed using IBM SPSS Statistics 20. P value was calculated using McNemar's test. Evaluation of diagnostic accuracy of trichoscopic variables was performed using “Medcalc ® diagnostic test evaluation calculator” available from the Internet as free statistical calculator.
The prevalence of FPHL in India is unknown. However, community-based surveys from China and Korea had shown a prevalence of 6% and 5.6%, respectively.[16] Thus, a population prevalence of 6% was considered for the sake of sample size calculation. Sample size calculation was performed according to a method described by Daly and Bourke.[17] The proportion of controls having the disease thus is expected to be 6% and we wished the study sample to be large enough to have a 90% chance of detecting a difference of 10% in cases, using a 5% two-sided level of significance. The sample size using the above method and considerations came out to be 201 subjects in each group.
To avoid interobserver bias, all the images were analyzed by the same investigator.
Results
A total of 4368 females with the complaint of hair loss presented during the study period. A total of 230 subjects in each group were recruited. Images from subjects having the presence of scaling or colored scalp skin or blurred images were rejected. Finally, the data obtained from 230 cases and controls were analyzed. Baseline characteristics of study subjects are given in [Table - 3].

Trichoscopic findings over the frontal area and the occipital area of cases and control group are described in [Table - 4].
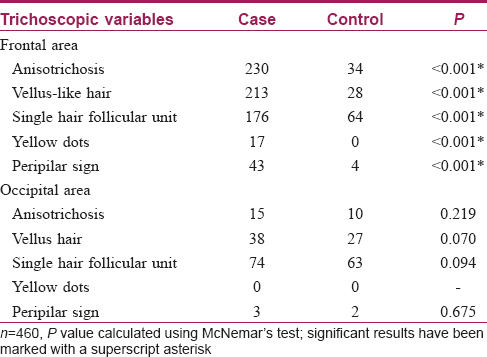
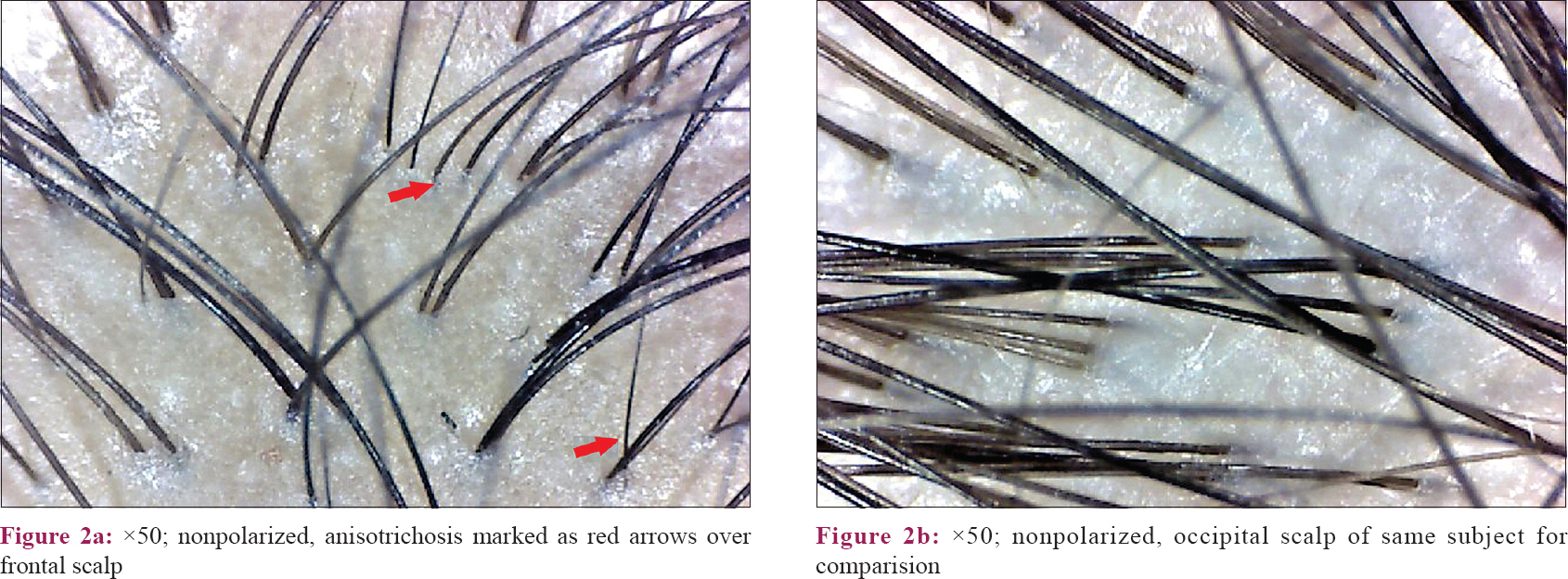
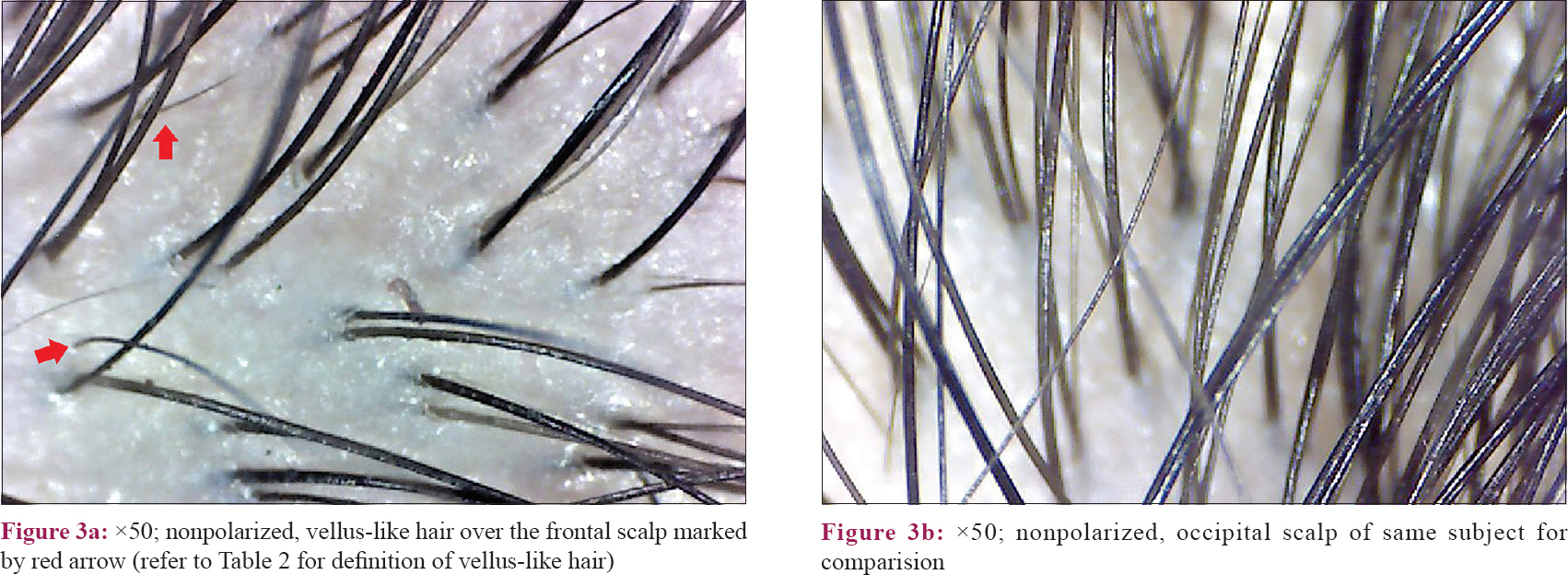
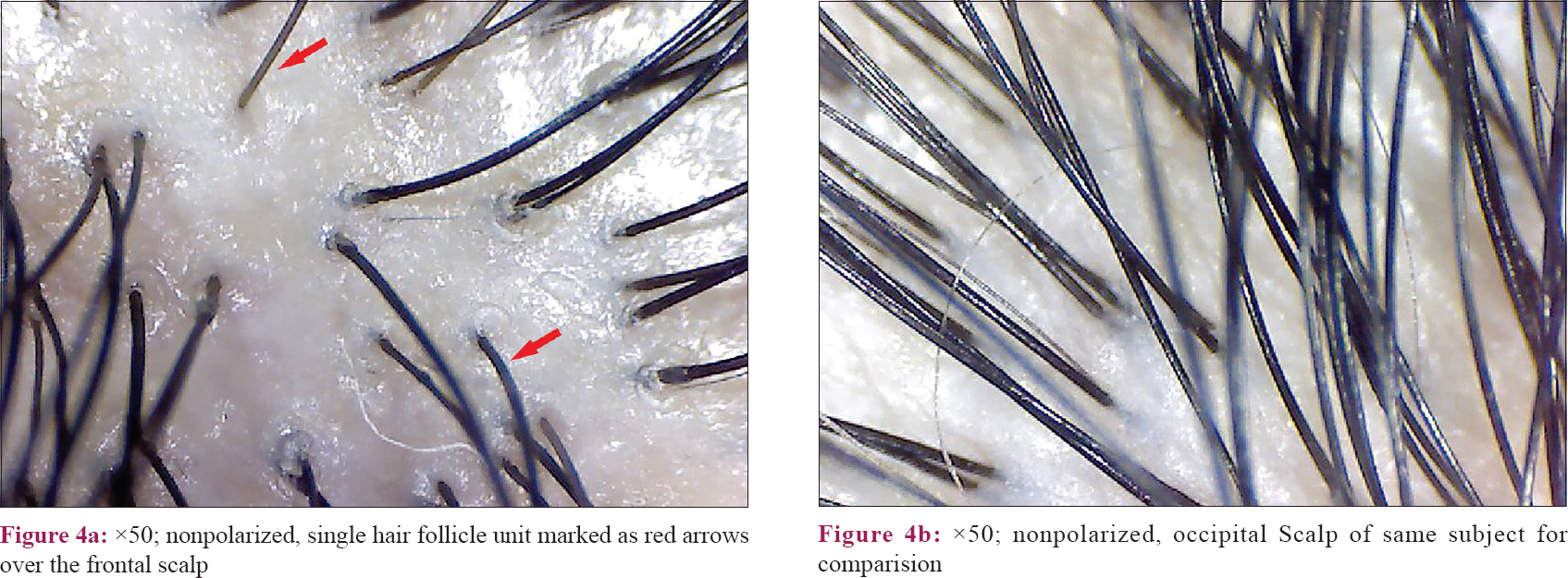
All the cases in the frontal area showed anisotrichosis [Figure - 2]a and an increase in the number of vellus-like hairs [Figure - 3]a, when compared to their own occipital scalp [Figure - 2]b, [Figure - 2]3b. Hair follicle unit containing a single hair [Figure - 4]a was also observed in a significant number of cases in the frontal area (as above, when compared to their own occipital scalp [Figure - 4]b).
 |
| Figure 2: |
 |
| Figure 3: |
 |
| Figure 4: |
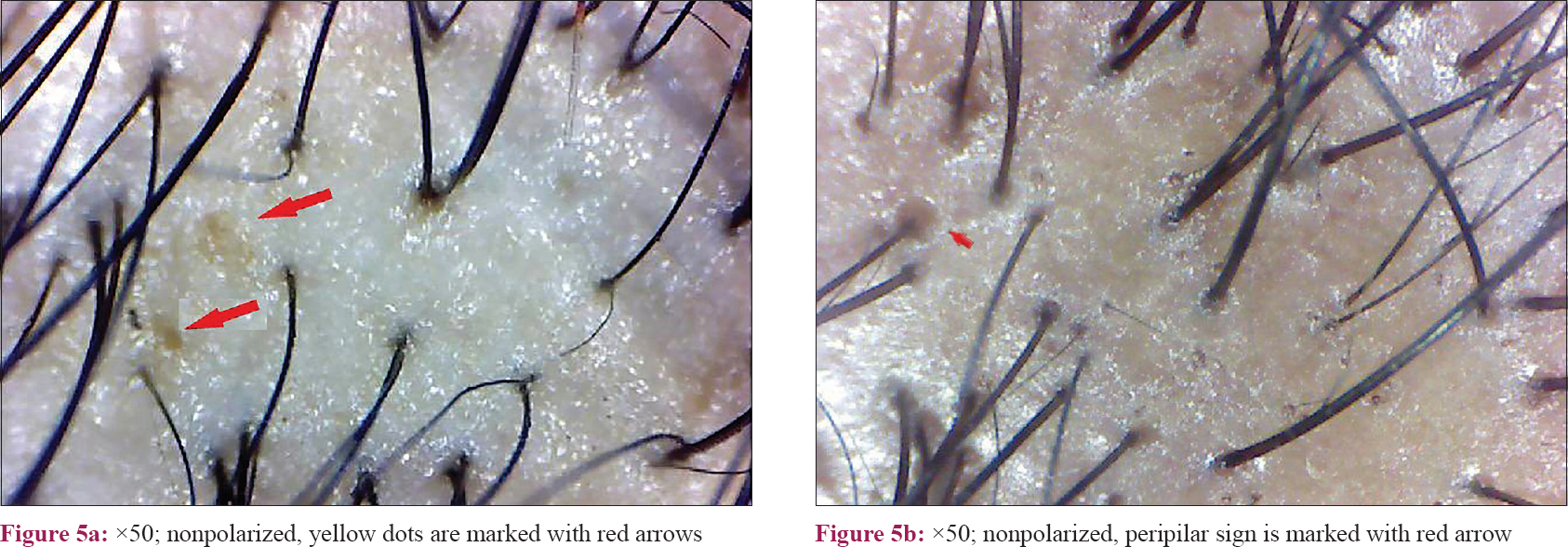
However, yellow dots and peripilar sign [Figure - 5] were noticeable in fewer study subjects. This is because non polarized dermoscopy was done, where these findings are not well seen. Hence, this finding does not have significance in this study.
 |
| Figure 5 |
Similar findings were also observed in the frontal area of controls but less pronounced.
Dermoscopic changes defined by the presence of anisotrichosis, vellus hair, single hair follicular unit, peripilar sign and yellow dots were found to be significantly associated with the frontal scalp of cases when compared with the frontal scalp of controls. Also, the frontal scalp of cases differed significantly from their own occipital areas. However, changes between occipital areas of cases and controls were not significantly different. Furthermore, the occipital area of the controls did not differ significantly from their frontal area. Thus, we may deduce that dermatoscopic findings mentioned above are significantly associated with the frontal scalp of cases only.
Discussion
In our study, we have recognized the changes in early FPHL using a low-cost, easy, and rapidly usable USB dermoscope. We have defined early FPHL on the basis of duration of the subjective complaint and the width midline hair parting, which were 6 months and 1 cm, respectively.
In a recent study, Rakowska et al. have established the trichoscopic criteria to diagnose FPHL.[7] However, diagnoses using these parameters require measurements performed through videodermoscope. Such instruments are costly and may not be available to most of the dermatologists, especially in resource-poor setting. As these criteria have already been established, we wanted to use them for the purpose of our study and at the same time, we wanted to assess these parameters using a simple dermoscope. Thus, we modified the parameters laid down by Rakowska et al. and recorded them using a simple dermoscope in the manner described in [Table - 2].[7]
Variation in hair shaft diameter is the most common feature observed in FPHL. This finding is also termed as anisotrichosis.[12] It classically reflects hair miniaturization due to disease (this feature correlates well with histopathological picture). Previous studies have described anisotrichosis of >20% hair follicles as significant of FPHL.[7],[13],[18] This finding was present in the frontal scalp of all the cases in our study. Also, there is an increased proportion of thin hair observed in the frontal area relating to hair miniaturization. Bhamla et al.[10] in their study had compared trichoscopic changes in females with early hair loss (<6 months) with groups of healthy controls and with late stages of the disease. However, they used a single trichoscopic parameter of hair diameter diversity (anisotrichosis), and histopathological correlation was also done. In this study, the case group comprised patients of FPHL and chronic telogen effluvium, and two patients of indeterminate histopathological diagnosis. They used only a single parameter of hair diameter diversity and suggested that trichoscopy could diagnose 75% of grade I FPHL when this parameter is present in more than 20% of hair. In our study, while comparing the frontal scalp with occipital scalp of controls, we have found that anisotrichosis achieved statistical significance [Table - 5] suggesting healthy females may also have significantly increased hair diameter variability in the frontal scalp. Thus, until an exact percentage of anisotrichosis has been calculated or until a suitable reference (like occipital area) is assessed, this parameter alone may not be a good tool for screening.
The normal hair follicular unit comprises one to four terminal hair with one to two vellus hair. However, the frontal scalp of cases showed an increased number of hair follicles containing single hair. Thus, increased thinning of hair and increase in the number of single hair follicular units with predominant prevalence in the frontal area are the main features observed in FPHL.
Previous studies have also described peripilar sign and yellow dots as the features of androgenic alopecia.[7] An approximately 1 mm brownish hyperpigmented halo around the follicular ostium reflects the presence of perifollicular lymphocytic infiltration, typical of the early stage of the disease.[15] However, in our study, it was found in only 43 (18.7%) cases in the frontal scalp. Similarly, another study done on Asian population showed that perifollicular pigmentation was less appreciated on the Asian scalp compared with the white population.[19] Yellow dots are the most common feature of alopecia areata, but it may be present in a wide spectrum of hair diseases, and thus it is not very specific for FPHL.[20] These dots represent empty follicular ostium and persistent sebaceous gland even after severe miniaturization of the follicles.[15] We found yellow dots in 17 (7.4%) cases suggesting this change is rare in the early stage of the disease. However, our findings should be interpreted with caution here since we used non-polarized dermoscopy in our study.
In our study, we found that hair-related trichoscopy changes, when assessed together, were significantly associated only with the frontal scalp of the patient suffering from early FPHL [Table - 6]. Similarly, the occipital area of the patients suffering from FPHL was not different from healthy subjects. Thus, in an individual having FPHL, their own occipital area can be used to compare frontal area, for assessing dermoscopically [Table - 6].
Limitations
We consider that our study is limited by the absence of histopathological evaluation of our cases. However, several previous studies have already confirmed the relationship between histopathological and dermoscopic parameters of FPHL. Also, recruiting patients of telogen effluvium as one of the control groups would have been desirable. However, it is difficult to diagnose and differentiate telogen effluvium by trichoscopy.[11]
Telogen effluvium has no specific trichoscopic criteria, even less so in early stages of the disease; thus we have excluded such cases on clinical grounds.[11]
To summarize, our study has found that even in early stages of FPHL, frontal scalp can be differentiated from the occipital scalp using a simple dermoscope.
Also, the hair changes, which we have studied, were able to differentiate the frontal scalp finding of early FPHL from normal control. Our study finding suggests that the occipital area in cases and controls have similar dermoscopic picture. So, in cases of early FPHL, the occipital area may serve as a reliable comparison zone for analysis of frontal scalp by dermoscopy.
Conclusion
From the above study, it can be concluded that a dermoscope is an excellent tool for the preliminary evaluation of females with history of hair loss as recent as 6 months. Moreover, diagnosis of early FPHL can be suggested using a dermoscope incapable of taking precise hair related measurements. Dermoscopic changes in the form of anisotrichosis, vellus-like hair, single hair follicular unit, peripilar sign and yellow dots, when taken together, may be used for diagnosis of early FPHL, provided the frontal scalp of the subject has these changes and the occipital scalp does not.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977;97:247-54.
[Google Scholar]
|
| 2. |
Sinclair R. Diffuse hair loss. Int J Dermatol 1999;38 Suppl 1:8-18.
[Google Scholar]
|
| 3. |
Olsen EA. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J Am Acad Dermatol 1999;40:106-9.
[Google Scholar]
|
| 4. |
Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci 1951;53:708-28.
[Google Scholar]
|
| 5. |
Harries M, Tosti A, Bergfeld W, Blume-Peytavi U, Shapiro J, Lutz G, et al. Towards a consensus on how to diagnose and quantify female pattern hair loss-the 'female pattern hair loss severity index (FPHL-SI)'. J Eur Acad Dermatol Venereol 2016;30:667-76.
[Google Scholar]
|
| 6. |
Rudnicka L, Rusek M, Borkowska B. Introduction. In: Rudnicka L, Rusek M, Borkowska B, editors. Atlas of Trichoscopy Dermoscopy in Hair and Scalp Disease. 1st ed. London: Springer-Verlag; 2012. p. 1-10.
[Google Scholar]
|
| 7. |
Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichology 2009;1:123-30.
[Google Scholar]
|
| 8. |
Price VH. Androgenetic alopecia in women. J Investig Dermatol Symp Proc 2003;8:24-7.
[Google Scholar]
|
| 9. |
Hung PK, Chu TW, Tsai RY, Kung CW, Lin SJ, Chen CM Quantitative assessment for female pattern hair loss. Dermatol Sin 2015;33:142-5.
[Google Scholar]
|
| 10. |
Bhamla SA, Dhurat RS, Saraogi PP. Is trichoscopy a reliable tool to diagnose early female pattern hair loss? Int J Trichology 2013;5:121-5.
[Google Scholar]
|
| 11. |
Lacarrubba F, Micali G, Tosti A. Scalp dermoscopy or trichoscopy. Curr Probl Dermatol 2015;47:21-32.
[Google Scholar]
|
| 12. |
Sewell LD, Elston DM, Dorion RP. “Anisotrichosis”: A novel term to describe pattern alopecia. J Am Acad Dermatol 2007;56:856.
[Google Scholar]
|
| 13. |
Herskovitz I, de Sousa IC, Tosti A. Vellus hairs in the frontal scalp in early female pattern hair loss. Int J Trichology 2013;5:118-20.
[Google Scholar]
|
| 14. |
Ramos LD, Santili MC, Bezerra FC, Ruiz Mde F, Petri V, Patriarca MT, et al. Dermoscopic findings in female androgenetic alopecia. An Bras Dermatol 2012;87:691-4.
[Google Scholar]
|
| 15. |
Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res 2004;295:422-8.
[Google Scholar]
|
| 16. |
Singal A, Sonthalia S, Verma P. Female pattern hair loss. Indian J Dermatol Venereol Leprol 2013;79:626-40.
[Google Scholar]
|
| 17. |
Daly LE, Bourke GJ, editors. Sample size determination. In: Interpretation and Uses of Medical Statistics. 5th ed. London: Blackwell Science; 2000. p. 269-95.
[Google Scholar]
|
| 18. |
de Lacharrière O, Deloche C, Misciali C, Piraccini BM, Vincenzi C, Bastien P, et al. Hair diameter diversity: A clinical sign reflecting the follicle miniaturization. Arch Dermatol 2001;137:641-6.
[Google Scholar]
|
| 19. |
Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol 2009;36:82-5.
[Google Scholar]
|
| 20. |
Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol 2006;55:799-806.
[Google Scholar]
|
Fulltext Views
9,576
PDF downloads
2,334





