Translate this page into:
“Verrucous vulva”: Meeting therapeutic challenges in massive condyloma acuminata with intralesional immunotherapy
Correspondence Address:
Saurabh Singh
Dermatology OPD, Main OPD Block, All India Institute of Medical Sciences, Basni Phase-2nd, Jodhpur - 342 005, Rajasthan
India
| How to cite this article: Singh S. “Verrucous vulva”: Meeting therapeutic challenges in massive condyloma acuminata with intralesional immunotherapy. Indian J Dermatol Venereol Leprol 2020;86:456-458 |
Sir,
Mycobacterium indicus pranii vaccine was developed and approved in India as an adjuvant to multidrug treatment of multibacillary leprosy for faster and complete immunological clearance of Mycobacterium leprae. Of late, it had been shown to be of benefit in anogenital warts.[1],[2]
A 57-year-old post-menopausal woman presented with progressive coalescent verrucous papules on the vulva since 1 year. Lesions started as asymptomatic skin-colored papules on the vulva a year back and gradually proceeded to cover most part of the visible genitalia. There was associated pruritus but no discharge. She was married but had no marital or extramarital sexual contact in the past 1 to 1.5 years and denied similar genital lesions in her husband. Her past medical history was unremarkable. Lesions had failed to respond to topical applications including salicylic acid, trichloroacetic acid, and 5-fluorouracil. Examination revealed a bulky labia majora with an uneven lobulated surface which was completely covered with skin-colored to grayish-white confluent verrucous papules and plaques [Figure - 1]a. On separating the labia majora, the verrucous lesions were found to diffusely involve and obliterate the labia minora and the clitoris was almost obscured [Figure - 1]b. Few scattered lesions were present in the perineal and perianal regions. Moderate non-foul smelling whitish discharge was visible at the introitus and per speculum examination showed features suggestive of vaginal candidiasis. Serology for sexually transmitted infections like enzyme-linked immunosorbent assay for human immunodeficiency virus, venereal disease research laboratory test and hepatitis B surface antigen and anti-hepatitis C virus antibodies were negative. Routine blood counts and biochemistry were also within normal limits. A diagnosis of extensive vulval condyloma acuminata with vaginal candidiasis was made. After treating vaginal candidiasis with oral fluconazole and clotrimazole vaginal pessary, the extensive vulval condyloma acuminata were planned for intralesional immunotherapy with Mycobacterial indicus pranii (MIP) vaccine. After a standard sensitizing test dose, 0.1 ml of the vaccine was injected in 2 wart sites in superficial dermis at 2 weekly intervals.[1] Patient achieved about 50–60% remission after 1 injection, 80–90% after 3 injections, and 95–100% after 4th and final injection [Figure - 2]a and [Figure - 2]b. Transient injection site tender nodules were noted on vulva [Figure - 2]b which disappeared spontaneously in next 7 to 10 days. The patient achieved complete remission at 10 weeks after starting therapy. The injection was associated with mild pain but no other adverse effects were noted. Patient reported being lesion free for 2 years after which she was lost to follow-up.
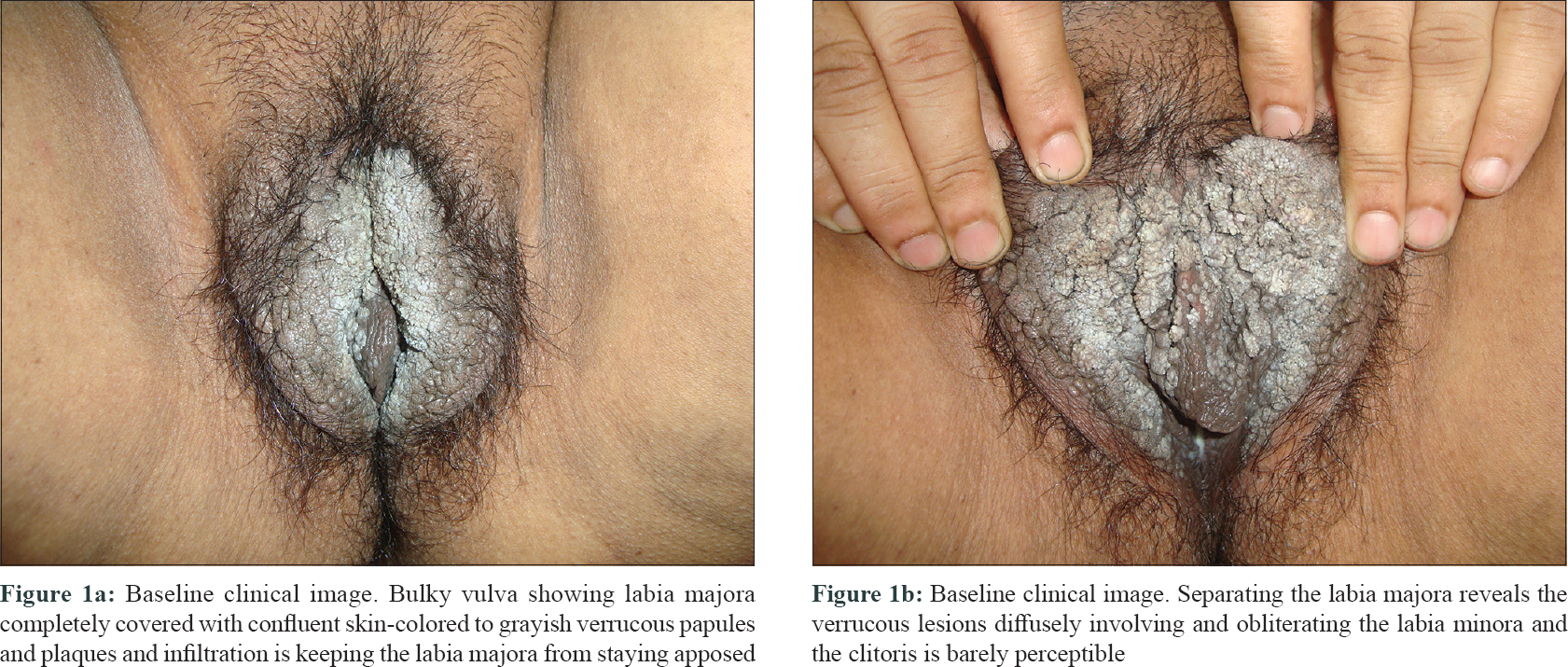 |
| Figure 1: |
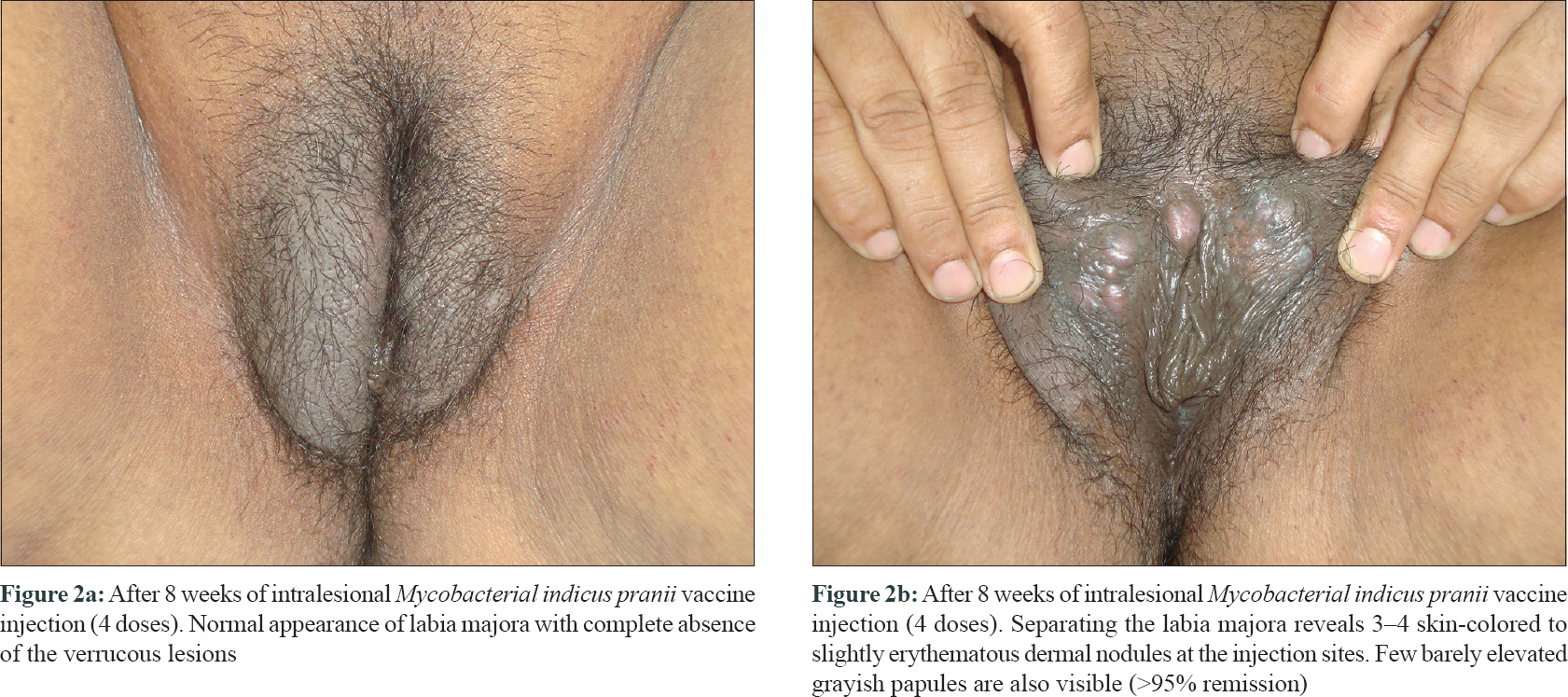 |
| Figure 2: |
The high immunogenicity of MIP vaccine has been found to have wider applications in fields like infectious diseases as well as oncology, the latest being its approval by drug controller general of India in 2013 for the treatment of advanced non-small cell lung cancer along with chemotherapy. The health ministry and Indian Council of Medical Research have recently initiated field trials of MIP vaccine as a prophylactic agent against leprosy. Despite this fact, unfortunately, the vaccine is currently unavailable commercially in India in a multi-dose vial preparation as needed for wart treatment and this is currently the greatest hindrance for further research and therapeutics.
The early studies using MIP vaccine included a sensitizing dose of intradermal injections on both deltoids[2],[3],[4],[5] but a series has shown effective results without the intradermal doses.[6] This vaccine has been widely reported to be beneficial in both extensive anogenital and extra-genital warts.[2],[3],[4] Its published efficacy in series on genital warts ranged from 67% to 88%.[1],[2] A study by Kumar et al. revealed that MIP vaccine fared slightly better than imiquimod in terms of proportion of patients achieving complete remission (67% in MIP group and 59% in imiquimod group), even though the results were not statistically significant. They also noted that although both drugs were able to reduce the viral load of human papilloma virus type 6, only MIP reduced the viral load of HPV-11. The only 2 prior studies using MIP vaccine in genital warts have reported complete remission, in one study in combination with vehicle cream after a mean of 5.66 doses (53.3 weeks)[2] and another study reporting time to remission ranging from 2 to 12 weeks.[1] Thus, the effects of vaccine may be delayed and may continue long after the treatment is over. Although the exact mechanism by which it works in warts is unknown, the evidence suggests that it mounts a strong Th1 cytokine response leading to effective T-cell and macrophage activation probably leading to infection clearance.[7]
The current case is being reported to highlight the rare instance of massive condyloma acuminata resolving completely and rapidly with intralesional MIP vaccine monotherapy. Despite the fact that several vaccines have been used to clear warts, the low cost, early response, and acceptable safety profile of killed MIP vaccine does indicate its suitability as an immunotherapeutic agent for resistant extensive warts. It is hoped that scientific literature will provide the much-needed impetus for the Government of India and pharmaceutical industry to reinitiate the process of making MIP vaccine commercially available for prescription usage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol 2008;22:1089-93.
[Google Scholar]
|
| 2. |
Kumar P, Dar L, Saldiwal S, Varma S, Datt Upadhyay A, Talwar D, et al. Intralesional injection of Mycobacterium w vaccine vs. imiquimod, 5%, cream in patients with anogenital warts: A randomized clinical trial. JAMA Dermatol 2014;150:1072-8.
[Google Scholar]
|
| 3. |
Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol 2013;149:237-9.
[Google Scholar]
|
| 4. |
Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol 2014;80:509-14.
[Google Scholar]
|
| 5. |
Khullar G, Narang T, De D, Nahar Saikia U, Dogra S, Handa S. Recalcitrant giant condyloma acuminatum treated successfully with a novel combination of Mycobacterium indicus pranii immunotherapy and acitretin. Int J STD AIDS 2017;28:1155-7.
[Google Scholar]
|
| 6. |
Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg 2014;7:203-8.
[Google Scholar]
|
| 7. |
Aldahan AS, Mlacker S, Shah VV, Kamath P, Alsaidan M, Samarkandy S, et al. Efficacy of intralesional immunotherapy for the treatment of warts: A review of the literature. Dermatol Ther 2016;29:197-207. Sir,
[Google Scholar]
|
Fulltext Views
2,914
PDF downloads
1,147





