Translate this page into:
Vismodegib treatment in a HIV positive patient on antiretroviral therapy
Correspondence Address:
Alessia Villani
Department of Dermatology, University of Naples Federico II, Via Pansini 5, 80131, Naples
Italy
| How to cite this article: Scalvenzi M, Villani A, Mazzella C, Cappello M, De Fata Salvatores G, Costa C. Vismodegib treatment in a HIV positive patient on antiretroviral therapy. Indian J Dermatol Venereol Leprol 2018;84:758-760 |
Problem
A 64-year-old man presented with a 3-year history of ulcerative basal cell carcinoma involving the auricular area and extending to the middle ear [Figure - 1]. The patient was diagnosed as human immunodeficiency virus (HIV) positive in 1988 and was under antiretroviral therapy with emtricitabine, rilpivirin and tenofovir with good control of his virological status. Basic investigations were within normal limits and skin biopsy from the affected site confirmed the diagnosis of basal cell carcinoma. An otolaryngologist was consulted and a computerized tomography of the head was taken to exclude any temporal bone or parotid gland involvement. Though the skin tumor had not affected the auricular nerve, due to its extension, an invasive surgery was required. However, the patient, who was a musician by profession, refused any surgical intervention. Hence, the basal cell carcinoma was treated with topical application of 5-fluorouracil ointment for 4 weeks with partial improvement of the lesion. However, recurrence occurred after one month.
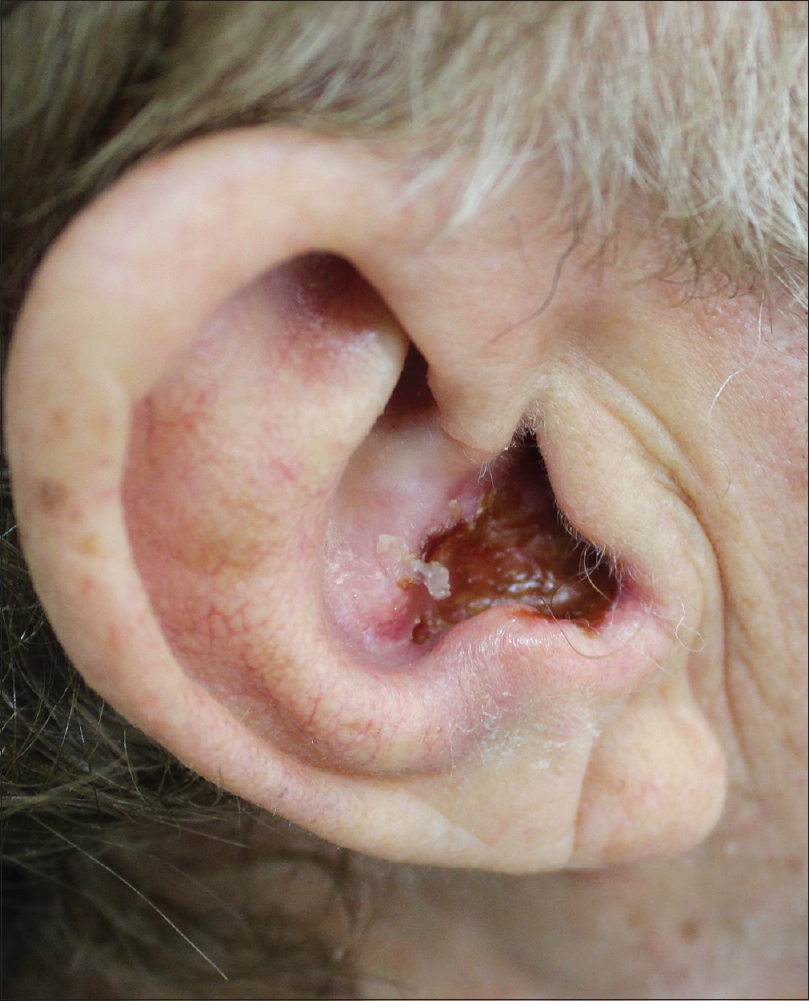 |
| Figure 1: Ulcerated basal cell carcinoma of the ear concha in a HIV positive patient |
Solution
As an alternative approach, after consulting the infectious disease specialist and an accurate evaluation of CD4 lymphocyte count (1250/μl), the patient was prescribed oral vismodegib (150 mg daily) at lunch time, before meal. Complete resolution of the skin disease on clinico-dermoscopic and histopathological examination was achieved after 24 weeks of therapy after which vismodegib was discontinued [Figure - 2] and [Figure - 3]a and [Figure - 3]b. For the entire duration of the treatment, the patient was on a 2-monthly follow-up. The therapy was well tolerated and CD4 count remained within the normal limits (1200/μl). During therapy, the patient showed typical side effects of the drug such as dysgeusia, asthenia and muscle cramps. No interactions between vismodegib and the antiretroviral drugs were observed. Currently, the patient is still on follow-up every 4 weeks since 1 year and no recurrence of the basal cell carcinoma has been observed. People with HIV have a 2.8-fold higher risk than HIV-uninfected persons of nonmelanoma skin cancer. Basal cell carcinoma is the most common skin cancer among immunocompetent individuals and accounts for 80% of all malignant cutaneous lesions.[1] However, among immunocompromised persons, including organ transplant recipients and persons with immune dysfunction, squamous cell carcinomas predominate over basal cell carcinomas.[2] Although Mohs micrographic surgery is the first-line therapy for basal cell carcinoma, some of them can progress to an advanced or, rarely, a metastatic state, so that non-surgical approaches, such as cryotherapy, adjuvant radiotherapy and targeted therapy are required. Vismodegib, an oral sonic hedgehog signaling pathway inhibitor, has been recently approved by the Food and Drug Association for the treatment of locally advanced, metastatic and recurrent basal cell carcinomas that are inappropriate for surgery or radiotherapy, representing a valid therapeutic alternative.[3],[4] Vismodegib selectively connects to the smoothened protein and blocks intracellular signaling, and thus, deactivates the hedgehog pathway, resulting in tumor growth inhibition. Muscle spasm, alopecia, dysgeusia and weight loss are the most common side effects described in the literature. Treatment is pursued until complete regression of the tumor. We were unable to find any previous report describing the use of hedgehog signaling pathway inhibitor in HIV positive patients treated with antiretroviral drugs. The safety of this drug in immunocompromised patients and possible interactions with other drugs are not reported in the vismodegib data sheet. In 2015, Otsuka et al. suggested that the use of hedgehog pathway inhibitors may create an alteration of the immune system, resulting in activation of the adaptive immune functions.[5] Although vismodegib appears to be a safe drug for the treatment of inoperable locally advanced or metastatic basal cell carcinomas, in high-risk patients, the optimal treatment protocol is still unknown; further studies are required to better evaluate the use of this targeted therapy for basal cell carcinomas, as an alternative approach in HIV positive inoperable patients.
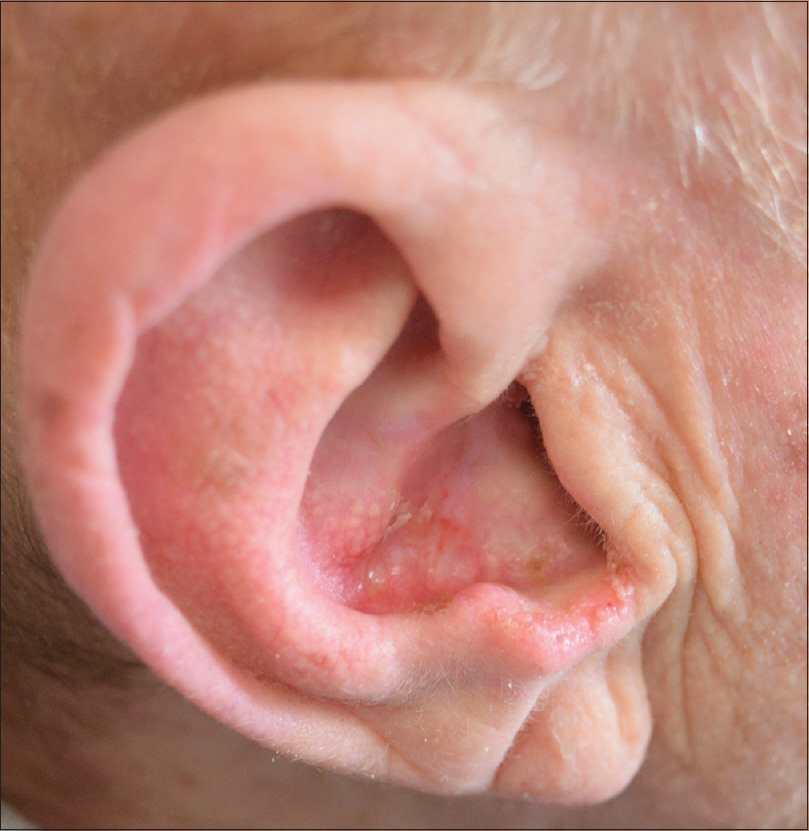 |
| Figure 2: The patient after 24 weeks of vismodegib therapy: Absence of diagnostic clinical manifestations of basal cell carcinoma |
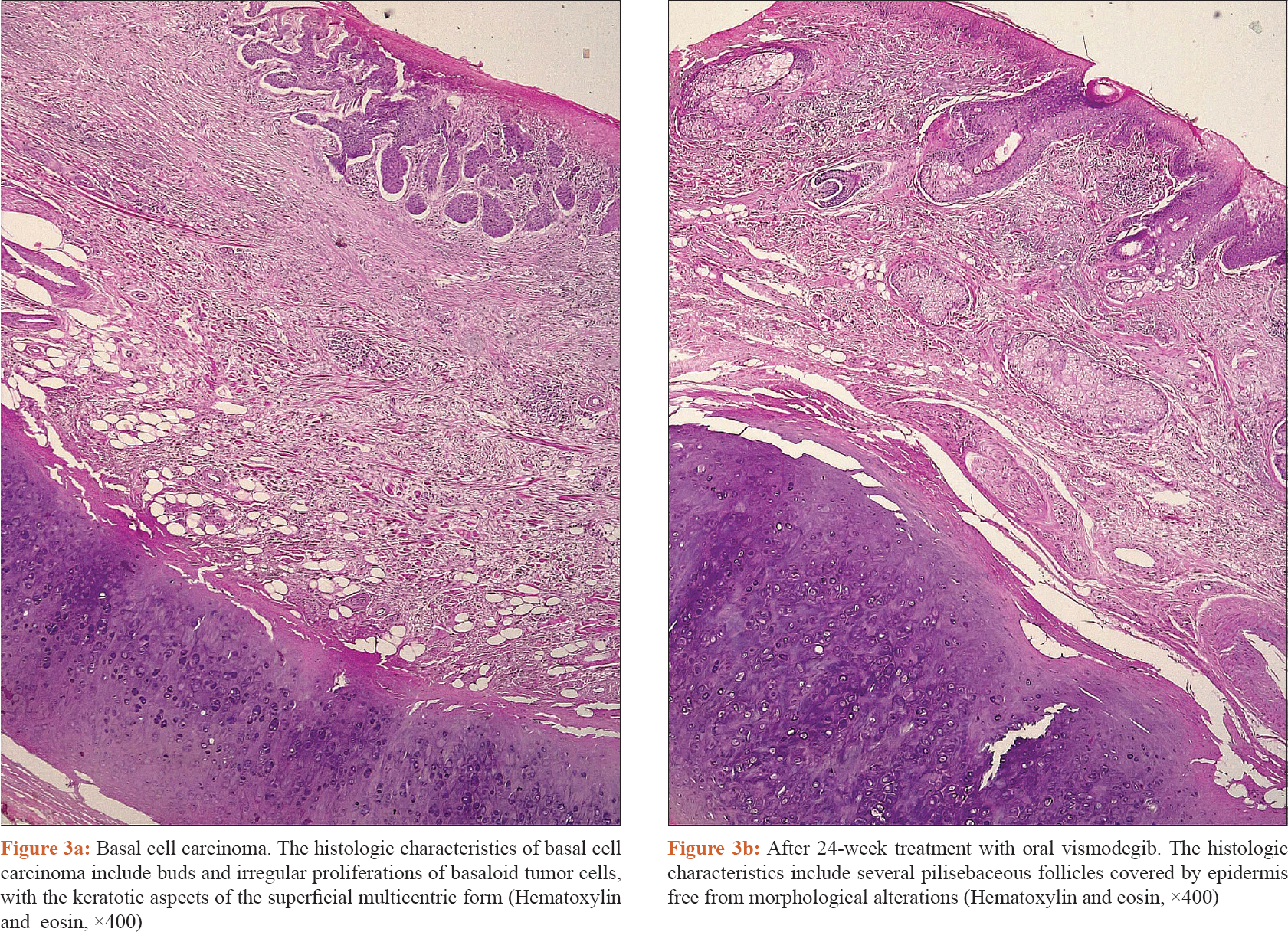 |
| Figure 3: |
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initial will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Basset-Séguin N, Hauschild A, Kunstfeld R, Grob J, Dréno B, Mortier L. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur J Cancer 2017;86:334-48.
[Google Scholar]
|
| 2. |
Asgari MM, Ray GT, Quesenberry CP Jr., Katz KA, Silverberg MJ. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol 2017;153:892-6.
[Google Scholar]
|
| 3. |
Danhof R, Lewis K, Brown M. Small molecule inhibitors of the hedgehog pathway in the treatment of basal cell carcinoma of the skin. Am J Clin Dermatol 2018;19:195-207.
[Google Scholar]
|
| 4. |
Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012;366:2171-9.
[Google Scholar]
|
| 5. |
Otsuka A, Dreier J, Cheng PF, Nägeli M, Lehmann H, Felderer L, et al. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin Cancer Res 2015;21:1289-97.
[Google Scholar]
|
Fulltext Views
3,185
PDF downloads
2,174





