Translate this page into:
Vitamin D and skin diseases: A review
Correspondence Address:
Vineet Relhan
35-F, Sector-7, SFS Flats, Jasola Vihar, New Delhi - 25
India
| How to cite this article: Wadhwa B, Relhan V, Goel K, Kochhar AM, Garg VK. Vitamin D and skin diseases: A review. Indian J Dermatol Venereol Leprol 2015;81:344-355 |
INTRODUCTION
Vitamin D, also known as the sunshine vitamin, has been recently implicated in a plethora of medical illnesses. Decrease in incidence of rickets after fortification of foods with vitamin D led physicians to believe that vitamin D related health disorders had come to an end. But unfortunately, rickets appears to be a mere drop in the vast ocean of disorders resulting from vitamin D deficiency.
Adequate sunlight and a diet rich in oily fish and fortified milk are the major sources of vitamin D for humans. Vitamin D functions like a hormone and regulates parathyroid hormone (PTH), calcium and phosphorous metabolism leading to important implications for the integrity of the skeletal system. The discovery of vitamin D receptors (VDRs) in most cells of the body and the presence of enzymes that synthesize the active form of vitamin D, namely 1,25-dihydroxyvitamin D [1,25(OH) 2 D] in non-renal sites like skin have led to a renewed interest in its functions, [1] particularly its role in decreasing the risk of several chronic, highly morbid conditions such as carcinomas, autoimmune diseases, infectious diseases, and cardiovascular diseases. [1]
The cutaneous synthesis of vitamin D and its role in the treatment of certain common skin disorders like psoriasis has made it an important topic for dermatologists. Studies that point towards a role of vitamin D as an immunomodulator have opened channels to discover its therapeutic effects in atopic dermatitis, psoriasis, and skin cancer. [2]
The Cutaneous Biosynthesis Pathway of Vitamin D3
Pro-vitamin D3 or 7-dehydrocholestrol, which is primarily found in the basal and spinous cell layers of the epidermis, undergoes a photochemical reaction to form pre-vitamin D3. The UV light blocking function of melanin leads to a requirement of greater UV light exposure in order to produce equivalent amounts of Vitamin D3 in dark skinned populations. [Figure - 1] shows the vitamin D3 pathway in human skin. [3] In addition to its action on the kidneys, calcitriol bound to vitamin D binding protein acts by both genomic and non-genomic mechanisms on certain other target tissues like bone, intestine, and parathyroid gland that express the vitamin D receptors. [3]
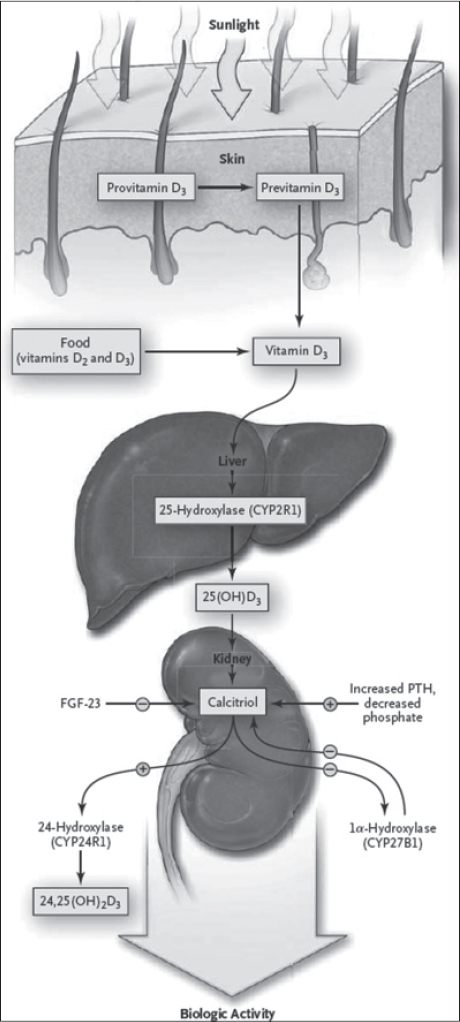 |
| Figure 1: Synthesis and metabolism of vitamin D |
Effects of Calcitriol on Cutaneous Biology
Calcitriol synthesized in the keratinocytes regulates their growth, differentiation, apoptosis and other biological processes via intracrine, autocrine and paracrine effects on the epidermal cell population. [4]
Effects on keratinocytes
Vitamin D leads to stimulation of in vitro keratinocyte proliferation at low concentrations and its inhibition at higher concentrations (≥10−8 M). [5]
Effects on skin immune system
Various immunological cells such as monocytes, T and B lymphocytes and Langerhans cells express both vitamin D receptor and 25-hydroxy-vitamin D-1α-hydroxylase [6] implicating a vital role of vitamin D in control and regulation of immune mechanisms. The immunomodulatory effects of vitamin D are summarized in [Table - 1].
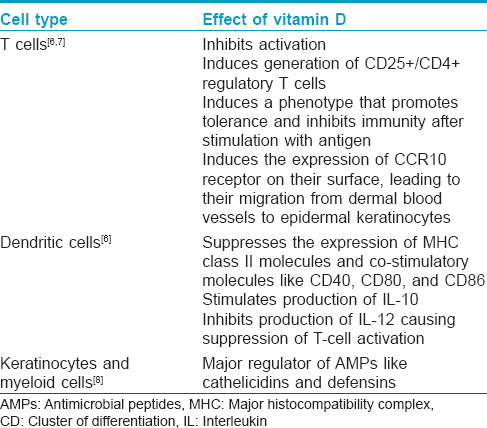
Effects on keratinocyte apoptosis
Calcitriol causes stimulation of ceramide synthesis by inducing the neutral Mg 2+ -dependent sphingomyelinase (thereby increasing the conversion of sphingomyelin to ceramide) [9] and in return, ceramide enhances the pro-differentiating effect of calcitriol on keratinocytes in a feedback loop. [10] Whereas pharmacological levels of calcitriol induce apoptosis of keratinocytes and other epidermal cells, [11] physiological levels block the effect of pro-apoptotic ceramides, UV radiation and tumor necrosis factor-α (TNF-α).
Effect as an antioxidant
The generation of an antioxidant in keratinocytes in vitro[12] may explain the photoprotection that vitamin D offers against harmful oxygen radicals induced by exposure to UVB.
Role of Vitamin D in Skin Diseases
Vitamin D and psoriasis linkage
In spite of the well established role of topical vitamin D analogs in psoriasis, the precise mechanisms underlying their therapeutic effectiveness are still not completely understood. Modulation of various markers of epidermal proliferation, proliferating cell nuclear antigen (PCNA) and Ki-67 antigen, and differentiation (involucrin, transglutaminase K, filaggrin, cytokeratins 10, 16) has been shown in situ in lesional psoriatic skin following topical application of vitamin D analogs. [13] However, topical vitamin D does not help much in reducing dermal inflammation (cluster of differentiation (CD-) antigens, cytokines, HLA-DR, etc) seen in psoriasis due to reduced bioavailability of topical preparations in the dermis. [13]
The therapeutic effects of topical vitamin D occur via a vitamin D receptor mediated genomic mechanism resulting in inhibition of keratinocyte proliferation. [14] Non-genomic mechanisms induce keratinocyte differentiation by increasing intracellular calcium levels. [15] Besides this, inhibition of T cell differentiation and proliferation in response to interleukin 1 (IL-1) by vitamin D decreases immunoglobulin production. The anti-inflammatory effects may also result from inhibition of production of IL-2, IL-6, and interferon-gamma (IFN-γ). Further, topical calcipotriol inhibits human beta defensin (HBD)2, HBD3, IL-17A, IL-17F, and IL-8 which are found in increased levels in psoriatic lesions, [16] thus blocking the cathelicidin pathway, reducing inflammation and reversing some of the changes that occur in psoriatic lesion.
The correlation of the clinical improvement with increased vitamin D receptor mRNA in vitamin D-treated cutaneous lesions, [17] has led to the categorization of "responders" and "non-responders" with the former showing an increase in vitamin D receptor mRNA in treated skin areas. [14] Allelic variations in individual vitamin D receptor genes may also determine response to treatment. An increased association of the A allele for the vitamin D receptor was found in patients with psoriasis. [18] According to Colin et al., the FokI polymorphism is associated with the response to calcipotriol. However, other studies have shown that distinct vitamin D receptor genotypes are not associated with clinical response to calcipotriol. [13] Studies on the serum levels of 1,25(OH) 2 D or 25(OH)D in psoriatic patients have shown conflicting results, with some showing reduced levels of 1,25(OH) 2 D in patients with manifest psoriasis. [19] Besides, the coincidence of pustular psoriasis with hypocalcemia [20] and exacerbation of psoriasis with chloroquin therapy (mediated via reduction in 1,25(OH) 2 D levels due to inhibition of 1α-(OH)ase (CYP27B1)) are well known. [21]
Improvement of psoriasis with sun exposure initiated the oral use of 1,25-dihydroxyvitamin D (calcitriol) as a therapeutic option. [13] In 1985, MacLaughlin et al. speculated that oral calcitriol may be effective in the treatment of psoriasis. [22] In the same year, Morimoto and Kumahara reported remission of psoriatic skin lesions in a patient of osteoporosis who was treated with oral 1α-(OH)D 3 . [23] Unfortunately, the oral use of vitamin D is limited due to the risk of adverse effects such as hypercalcemia, hypercalciuria, nephrocalcinosis, nephrolithiasis and reduction in bone mineral density secondary to its calcemic effect. [13] But a recent review shows that hypercalcemia can be easily monitored and is avoidable with proper dosing and monitoring. A correlation between low levels of serum vitamin D and increased severity of psoriasis has also been documented in the literature. [24] Further, with the growing evidence that psoriasis is a systemic disease that affects many organ systems and results in comorbidities, there is an urgent need for re-evaluation of the role of oral vitamin D in the treatment of psoriasis. Perez and colleagues [25] observed improvement with oral vitamin D in 88% of 85 patients with psoriasis; 26.5% had complete clearance, 36.2% had moderate improvement and 25.3% had slight improvement. Gaal et al. [26] observed a significant immunomodulatory effect of systemic alphacalcidol [1α-(OH)D 3 ] on patients with psoriatic arthropathy and proposed it as a valuable therapeutic alternative. Another study demonstrated that a combination of acitretin and oral calcitriol resulted in a faster reduction of PASI (Psoriasis Area Severity Index) in patients of chronic plaque psoriasis. [27] Published reports suggest that oral vitamin D through its anti-inflammatory action also improves the metabolic syndrome and cardiovascular disease which may be associated with psoriasis. [24] Larger studies should be undertaken with this inexpensive and easily available option in the management of psoriasis and the associated metabolic syndrome.
Topical calcitriol with its minimal effects on serum calcium has been approved by the US Food and Drug Administration (USFDA) for the treatment of psoriasis. The effectiveness and safety of various vitamin D analogs including calcitriol, calcipotriol, tacalcitol, hexafluoro-1,25-dihydroxyvitamin D3 and maxacalcitol in the topical treatment of psoriasis have been documented in several studies. [13] They are more efficacious with superior cosmetic acceptability than the earlier topical treatments such as tar and dithranol and have potency comparable to mid-potency topical corticosteroids. [28]
Lack of skin atrophy or adrenal suppression is another reason for their inclusion in the therapeutic armamentarium for psoriasis. [28]
The role of various available topical vitamin D analogs [28] is presented in [Table - 2]. Combined topical treatment with calcipotriol ointment (50 μg/g) and betamethasone ointment was shown to be less irritating and slightly more effective than calcipotriol used alone. [29] As a rule, the use of calcipotriol ointment on face and flexures should be avoided due to its irritancy. Scalp psoriasis responds well to calcipotriol solution without side effects. Therapeutically resistant areas like nails have been reported to have responded to calcipotriol ointment but there have been no consistently effective treatment for psoriatic nails up till now. [28]

Kragbelle and coworkers observed that topical application of calcipotriol ointment (any time upto 2 hours before or immediately after) with simultaneous UV-B phototherapy caused an improvement in the psoriatic lesions, possibly due to increased cutaneous vitamin D synthesis. [30] Combination therapy with agents like TNF-α inhibitors, methotrexate (MTX), low-dose oral cyclosporine (2 mg/kg/day), oral acitretin, topical dithranol and topical steroids may enhance the therapeutic efficacy of topical vitamin D. [31],[32],[33],[34],[35],[36]
Psoriasis is a chronic condition with frequent relapses and may require life long intermittent therapy.
It is now accepted that vitamin D analogs are effective and safe for the topical treatment of skin areas that are usually difficult to treat and that respond slowly. They do not exhibit tachyphylaxis and topical treatment can be continued indefinitely. Also, they are effective in the treatment of psoriatic skin lesions in children and in HIV patients. [28]
Vitamin D and atopic dermatitis
As vitamin D has the potential to suppress inflammatory responses, enhance antimicrobial peptide activity and promote the integrity of the permeability barrier, vitamin D supplementation provides a possible therapeutic intervention for atopic dermatitis. The role of vitamin D in the pathogenesis of atopic dermatitis has not been fully established. An observational study of 138 Norwegian patients with atopic dermatitis did not find any correlation between low dietary vitamin D and clinical disease severity scores. [37] A small pilot study showed a non-significant benefit of vitamin D supplementation in seasonal atopic dermatitis. [38] A study has shown that a higher intake of vitamin D in early life is associated with greater prevalence of atopic manifestations. This was in support of prior studies suggesting a role of vitamin D intake during infancy in the development of atopic allergy later in childhood. [39] On the contrary, some epidemiological studies have demonstrated that patients with atopic dermatitis have a lower vitamin D intake when compared with controls. [1]
Several recent reports have shown that vitamin D plays a role in the pathogenesis of atopic dermatitis through its immunomodulatory properties. Schauber et al. [40] demonstrated that the active form of vitamin D [1,25(OH) 2 D3] enhanced the expression of antibacterial peptides and thus prevented skin infections. Liu et al. [41] demonstrated a link between vitamin D-mediated activation of toll-like receptors, the production of cathelicidin and diminished sensitivity to bacterial infections. Depending on the concentration, vitamin D can stimulate or inhibit keratinocyte differentiation [6] and stimulate synthesis of proteins such as filaggrin that are necessary for formation of the stratum corneum barrier. [42] Further, it has been demonstrated that vitamin D analogs suppress in vitro immunoglobulin E (IgE) production and IgE-mediated cutaneous reactions. [43] Ong et al. demonstrated significantly decreased immunostaining for cathelicidins (LL-37) in acute and chronic atopic dermatitis lesions compared to psoriatic skin lesions. [44] This finding may explain the differences in skin infections between patients with these two diseases. Among patients with atopic dermatitis, those with a history of herpes simplex virus (HSV) superinfection have significantly lower cathelicidin levels. Antiviral assays have shown that cathelicidin has activity against herpes simplex virus. [45]
The various studies evaluating the role of vitamin D in atopic dermatitis have been tabulated in [Table - 3]. The results of these studies indicate that vitamin D supplementation may have a therapeutic role in the disease with a good safety profile. However, further trials involving larger sample sizes and longer treatment periods will be necessary to more fully assess vitamin D as a therapeutic strategy in atopic dermatitis.
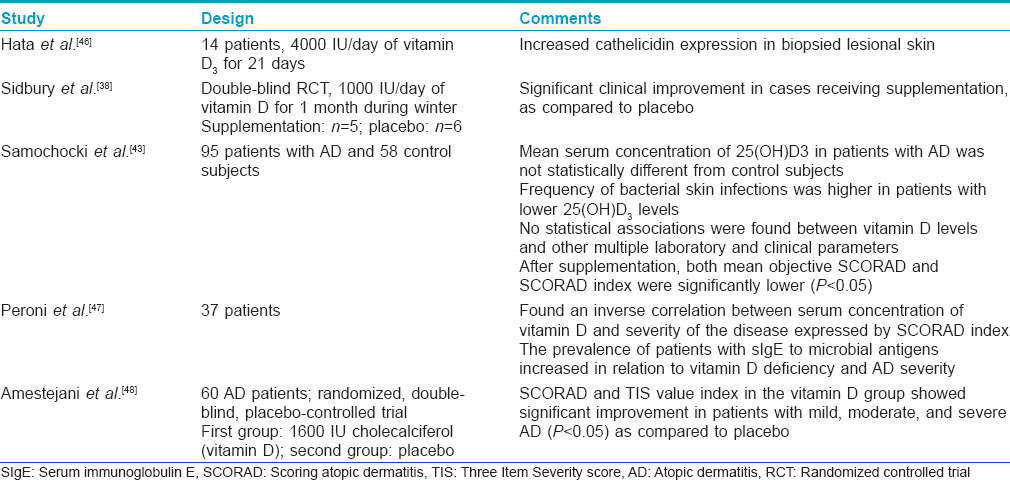
Vitamin D and vitiligo
Autoimmunity plays an important role in pathogenesis of vitiligo which is also evident by coexistence of various autoimmune disorders with vitiligo. [49] In turn, vitamin D levels have been found to be reduced in various autoimmune disorders, [50] thus pointing to a plausible association between vitiligo and vitamin D levels. However, the exact mechanism by which vitamin D effects autoimmunity is still an enigma.
Some studies have investigated the association between vitiligo and 25(OH)D levels. In the study by Silverberg et al. [51] decreased levels of 25(OH)D were found in patients with vitiligo with additional autoimmune diseases and in another study by Li et al., [52] statistically significantly decreased levels of 25(OH)D were seen in patients with vitiligo as compared to controls, thus suggesting that a deficiency in 25(OH)D may be a contributing factor in the development of vitiligo.
The use of vitamin D analogs in combination with PUVAsol for the treatment of vitiligo was first reported by Parsad et al. [53] Subsequently, topical vitamin D analogs have been effectively used in the treatment of vitiligo, both as monotherapy and in combination with other modalities such as corticosteroids and UV therapy. Studies have indicated that the target of this therapeutic intervention lies in melanocytes and the immune system. Vitamin D, through its antiapoptotic effect, controls the activation, proliferation and migration of melanocytes by increasing melanogenesis and the tyrosinase content of cultured human melanocytes. [54] Vitamin D also decreases the autoimmune damage of melanocytes by modulating T-cell activation. [55]
Several clinical trials have being conducted to evaluate the role of topical vitamin D in vitiligo, in combination with other therapies [Table - 4]. Since the existing clinical studies have shown variable efficacy of topical vitamin D, further studies are required to evaluate the exact relationship of serum vitamin D levels and vitiligo as well as to elucidate the effects of vitamin D in the treatment of vitiligo, either alone or in combination with other therapies. The role of oral vitamin D in the treatment of vitiligo has not been studied extensively. A pilot study was done to assess the efficacy and safety of prolonged high-dose vitamin D3 treatment of patients with psoriasis and vitiligo. This study was based on the fact that autoimmunity and vitamin D deficiency are related, and in turn vitamin D metabolism in affected patients is associated with the frequent presence of gene polymorphisms. Thus, giving high doses of vitamin D3 in patients with autoimmune disorders may compensate for inherited resistance to its biological effects. Finamor et al. treated 16 patients of vitiligo with 35,000 IU of vitamin D3 once daily for 6 months in association with a low-calcium diet and hydration. Fourteen of the 16 patients had 25-75% repigmentation with no significant change in metabolic parameters, thus indicating that high-dose vitamin D3 therapy may be effective and safe for vitiligo patients. [61]
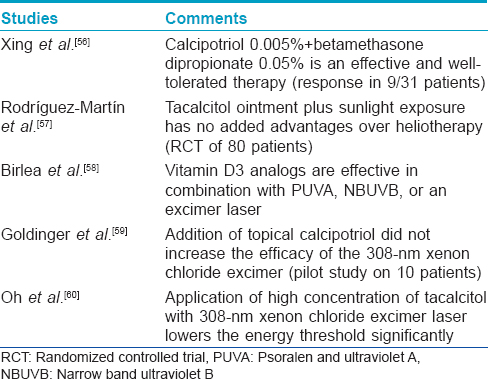
Vitamin D and ichthyosis
There are numerous reports of an association between disorders of keratinization and rickets in the literature. Calcitriol acts as an inhibitor of keratinocyte proliferation and also has a role in mineralization of new bones by increasing calcium and phosphorus absorption from the intestine. Thus, disorders like ichthyosis caused by abnormal keratinization may be associated with an alteration in vitamin D metabolism leading to rickets and osteomalacia. [62] Though it still needs to be determined whether this association is causal or coincidental, it is suggested that children with severe ichthyosis living in developing countries and especially those with pigmented skin should undergo an evaluation for rickets.
The various factors leading to rickets in a patient with disorder of keratinization may include alterations in epidermal cholesterol metabolism, avoidance of sunlight to prevent sunburn, associated vitamin D deficiency rickets, or increased keratinocyte proliferation leading to poor penetration of skin by sunlight. [63] Ingen-Housz-Oro et al. [64] reported severe vitamin D deficiency [25(OH)D levels below 25 nmol/l] in nine patients with severe phenotypes of congenital ichthyosis (lamellar ichthyosis in four and the less severe congenital ichthyosiform erythroderma phenotype in five), most of whom did not improve after the sunny season. The deficiency was more severe and parathyroid hormone (PTH) levels were elevated in patients of North African descent who had pigmented skin, as compared to European patients. However, these patients had normal levels of markers of bone formation and resorption, and normal 1,25(OH) 2 D levels despite reduced 25(OH)D levels. The authors postulated that severity of deficiency in their cases may be related to the severity of ichthyosis and skin pigmentation. All but one patient improved after summer months and he also did not have normal 25(OH)D values typically found in healthy subjects with similar sun exposure. Thus, it could not be definitely concluded whether the decreased vitamin D levels are a consequence of the keratinization disorder or are related to its physiopathology.
Sethuraman et al. [65] described five children with ichthyosiform erythroderma who had biochemical and radiological features of rickets. Clinically three out of these five patients showed signs of rickets, with severe skeletal manifestations in two. Another study of 15 patients with keratinisation disorders showed increased serum PTH levels with low to normal 25(OH)D levels in 5 patients, possibly reflecting a secondary hyperparathyroidism due to subtle calcium or vitamin D deficiency. [66] The findings may be a result of altered vitamin D metabolism due to disordered keratinisation, sun avoidance by patients, loss of calcium through skin or decreased calcium absorption due to retinoids ingestion. However, another study of 65 patients with keratinization disorders did not find any association between secondary hyperparathyroidism and vitamin D deficiency or retinoids intake. [67]
Though studies have shown an association between vitamin D levels and keratinization disorders, its therapeutic role is still unclear as significant improvement has not been documented in ichthyosis despite restoration of biochemical values and healing of rickets after oral vitamin D supplementation in patients with coexistent pathologies. [68],[69] However, one patient showed improvement in ichthyosis following topical treatment. Thus, additional studies are needed to establish a cause-benefit relation of vitamin D and ichthyosis and to determine whether topical calcipotriene used in treatment of ichthyosis can also prevent vitamin D deficiency in them.
Though most case reports are related to ichthyosis-induced vitamin D deficiency, Khateeb et al., reported a case of an 8-year-old child who had bullous ichthyosiform erythroderma with hypocalcemic vitamin D-resistant rickets (a specific type of rickets attributed to vitamin D receptor defect rather than to vitamin D deficiency). Although the association may be coincidental, the authors postulated that the association may also be due to close genetic localization of both conditions on the long arm of chromosome 12. [70]
There are several case reports from India which show an association between rickets and ichthyosis. Kothari et al. reported two cases of rickets due to vitamin D deficiency secondary to underlying ichthyotic skin disorder, both of whom improved with parenteral supplementation. [71] Kumar et al. reported rickets associated with autosomal dominant ichthyosis vulgaris. [72] In another cross-sectional study of 45 children and adolescents with ichthyosiform erythroderma and skin type IV and V, mean serum 25(OH)D was found to be significantly lower than in the control group and all patients had evidence of rickets. [73] Deka et al. also reported a case of lamellar ichthyosis with bilateral genu valgum. [74]
Thus, in developing countries like India, vitamin D level measurement should be done in a case of ichthyosis, as vitamin D deficiency is present in most Asian Indians due to skin pigmentation and inadequate direct sunlight exposure. Furthermore, in India, dairy products and food fats are not fortified with vitamin D, which, along with a low calcium and high phytate diet, is another contributing factor. [75]
Vitamin D and skin cancer
The finding of vitamin D receptor expression on both normal and malignant cells [76] initiated an investigation into the link between vitamin D and cancer. Ultraviolet radiation is a well known cause in the etiology of non melanoma cutaneous carcinomas. [3] As previously mentioned, vitamin D offers protection to keratinocytes in vitro against UV radiation. [77] Further, the development of cutaneous tumors in response to carcinogens in vitamin D receptor deficient mice has lead to the hypothesis that consistently raised serum vitamin D levels in response to chronic sun exposure protects against melanoma [2] Besides this, adequate serum vitamin D levels may decrease the incidence of solid organ tumors like those of stomach, liver, colorectum, gall bladder, pancreas, lung, breast, prostate, bladder and kidney, although the risk of non-melanoma cutaneous tumors is maintained due to the similar wavelengths required for vitamin D production and cutaneous photodamage. [2] Studies have also revealed a correlation between serum vitamin D levels and cancer severity, with higher vitamin D levels observed in stage I melanoma vs stage IV tumor. [78]
Promotion of cell differentiation and apoptosis along with inhibition of cancer cell proliferation, inflammation and angiogenesis by calcitriol may explain the cancer protective benefits of vitamin D. [79] Hence, oral vitamin D supplementation can be recommended to significantly reduce the cancer mortality from melanoma and other solid tumors without increasing the risk of non-melanoma cutaneous cancers inherent to increased ultraviolet light exposure. However vitamin D receptor gene polymorphisms observed in various studies may lessen the benefit of vitamin D in some patients. [80],[81],[82]
Vitamin D and skin fibrosis
In a study by Zhang et al.,[83] the finding of inhibition of normal skin fibroblasts and keloid fibroblasts by vitamin D has suggested a potential therapeutic role in keloids. Topical vitamin D analogs are an established treatment modality in morphoea and lichen sclerosus et atrophicus owing to its effects on immunoregulation, fibroblast proliferation, collagen synthesis and endothelial cell function. [84] Suppression of T lymphocytes by calcipotreine leading to decreased IL-2 secretion may also explain its beneficial effect in morphoea. [85],[86]
Application of calcipotriol ointment twice a day with overnight occlusion for 9 months was observed by Tay [87] to improve a plaque of morphoea in a 5-year-old child. Hulshof et al.,[88] reported improvement in joint mobility and skin extensibility in three patients of generalized morphoea after 3-7 months of treatment with 0.50-0.75 μg calcitriol oral daily without any untoward effects, which was maintained even after 1-2 years of discontinuation of therapy.
The basis for treating lichen sclerosus et atrophicus particularly extragenital disease, with vitamin D is similar to the rationale for its use in morphoea, although a precise mechanism of action has yet to be identified. [89]
Vitamin D and infections
Peacocke et al., [90] reported a single case demonstrating an association of vitamin D deficiency with desquamative inflammatory vaginitis and resolution of the vaginal discharge when the circulating concentration of vitamin D returned to the normal reference range. Low vitamin D levels have also been associated with bacterial vaginosis. [91]
Vitamin D and tuberculosis
The long standing observation of improvement in tuberculosis on exposure to sunlight was not just coincidental. Induction of antimicrobial peptides, mainly cathelicidins by vitamin D may support its beneficial effect against Mycobacterium tuberculosis. A reduction in the population of viable bacilli has been demonstrated when infected macrophages are treated with dihydroxycholecalciferol. [2] Yuk et al. showed that LL-37, induced by physiological levels of vitamin D, stimulates access to and killing of M. tuberculosis even within autophagosomes. [92] This explains the occurrence of low serum vitamin D levels in patients with current or prior M. tuberculosis infection. Combined vitamin D and antitubercular therapy has been observed to result in a higher degree of clinical improvement than the latter given alone. [2]
The use of calciferol for treatment of lupus vulgaris dates back to 1940s. A series of cases from England, France, Belgium and other countries showed that calciferol had an excellent curative effect on lupus vulgaris without causing much toxicity. [93] Nnoham and Clarke [94] did a systematic review and meta-analysis to explore the association between low serum vitamin D and the risk of active tuberculosis in humans. From the seven observational studies published between 1980 and July 2006 and identified through Medline that were finally included in this systematic review, it was concluded that low serum vitamin D levels are associated with a higher risk of active tuberculosis.
Vitamin D and autoimmune diseases
There are various reports of vitamin D receptor polymorphisms associated with increased risk of various autoimmune disorders like Hashimoto′s thyroiditis, inflammatory bowel disease, Graves′ disease, rheumatoid arthritis, systemic lupus erythematosus (SLE), primary biliary cirrhosis (PBC), autoimmune hepatitis, Addison′s disease, vitiligo, celiac disease, type I diabetes mellitus and multiple sclerosis (MS). [95] However these reports mainly involve in vitro and animal studies, with only conflicting data in humans. In spite of this, vitamin D supplementation has been proposed for the therapy of autoimmune disorders.
Other less-characterized roles of vitamin D
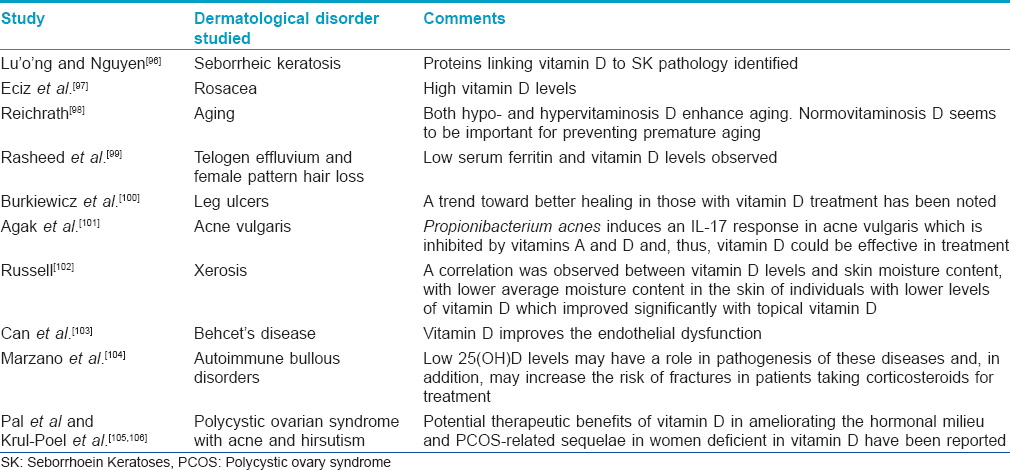
Other less-characterized roles of vitamin D have been summarized in [Table - 5].
EVALUATION OF NEW VITAMIN D ANALOGS
In view of the health benefits of vitamin D, multiple clinical and laboratory studies are underway to develop new vitamin D analogs with a better clinical profile. Some promising experimental compounds are: (1) analogs having predominant cutaneous metabolism thereby showing minimal systemic toxicity; vitamin D analogs, obtained by a combination of the 20-methyl modification with biologically interesting artificial side chain subunits or 2β-substituted calcitriols are promising candidates; (2) analogs with predominant cutaneous localization and without systemic side effects and (3) analogs with predominant effects on the target tissues and with minimal effects on calcium metabolism. This can be achieved by developing molecules showing variable affinities to vitamin D receptors and nuclear co-factors including retinoid X receptor α (RXRα).
Vitamin D in the Indian Scenario
There is a false belief that vitamin D deficiency is uncommon in India. The data available in the published literature show vitamin D deficiency in all age groups and both sexes across the country. [107],[108]
Vitamin D deficiency is considered to be present when serum 25(OH)D levels are <20 ng/ml, insufficiency when the level is between 20 and 30 ng/ml, and sufficiency when the level is >30 ng/ml. [108] This definition has been followed in most of the studies in India. The serum concentration of 25(OH)D may be effected by variables like age, sex, pubertal status, latitude, season, race, and ethnicity. [108]
Apart from low dietary intake causing vitamin D deficiency, people suffering from hepatic, renal and dermatological disorders, alcoholics and those suffering from inflammatory rheumatological conditions also have vitamin D deficiency. Other factors contributing to vitamin D deficiency in India are changing food fads and food habits, high-fiber diet, genetic factors (having increased 25(OH)D-24-hydroxylase which degrades 25(OH)D to inactive metabolites), more time spent indoors, increased pollution, cultural and traditional habits such as the use of "Burqa" in Muslims, and repeated and unplanned, un-spaced pregnancies in nutritionally deficient patients. [109]
According to Harinarayan et al., [110] factors like the solar zenith angle, time of day, season of the year, amount of ozone, cloud cover and latitude and altitude influence the amount of UVB radiation at a particular place, which in turn determines the amount of cutaneous vitamin D3 production. The Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Consultation [111] states that the abundant sunlight in most areas of the world between 42°N and 42°S latitude results in adequate cutaneous synthesis of vitamin D. Further, it proposes that thirty minutes of sun exposure over the arms and face in the absence of a sunscreen, preferably between 10 a.m. and 2 p.m. daily is adequate to avoid vitamin D deficiency.
Keeping in mind the enormous health benefits of vitamin D, guidelines for food fortification, vitamin D supplementation and adequate calcium intake for the Indian population should be formulated and implemented. As vitamin D insufficiency and deficiency are easily preventable, the current recommendations of taking 1-1.5 g of dietary calcium and 2000 IU of vitamin D per day in the diet should be adhered to avoid vitamin D deficiency. [110]
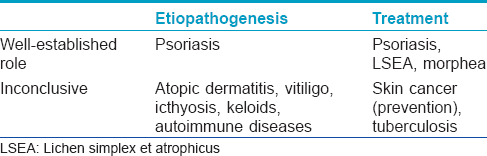
[Table - 6] summarizes the role of vitamin D in various skin disorders. Besides the role of vitamin D in the prevention of rickets and other bone disorders, interesting evidence has been unfolding on the relationship between vitamin D and other systemic diseases with many studies being undertaken to discover its other potential benefits though the data is still contradictory and insufficient for any clinical recommendations. Further studies are required to clarify the health promoting effects of vitamin D in humans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81.
[Google Scholar]
|
| 2. |
Miller J, Gallo RL. Vitamin D and innate immunity. Dermatol Ther 2010;23:13-22.
[Google Scholar]
|
| 3. |
Lehmann B, Querings K, Reichrath J. Vitamin D and skin: New aspects for dermatology. Exp Dermatol 2004;13(Suppl 4):11-5.
[Google Scholar]
|
| 4. |
Segaert S, Bouillon R. Epidermal keratinocytes as source and target cells for vitamin D. In: Norman AW, Bouillon R, Thomasset M, editors. Vitamin D Endocrine System: Structural, Biological, genetic and Clinical Aspects. Proceedings of the Eleventh Workshop on Vitamin D, Nashville, TN, USA: University of California, Riverside: Printing and Reprographics; 2000. p. 583-90.
[Google Scholar]
|
| 5. |
Gniadecki R. Stimulation versus inhibition of keratinocytes growth by 1,25-dihydroxy vitamin D3:Dependence on cell culture conditions. J Invest Dermatol 1996;106:510-6.
[Google Scholar]
|
| 6. |
Van Etten E, Decallone B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C, et al. Analogs of 1α,25-dihydroxy vitamin D3 as pluripotent immunomodulators. J Cell Biochem 2003;88:223-6.
[Google Scholar]
|
| 7. |
Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to "program" T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007;8:285-93.
[Google Scholar]
|
| 8. |
Dombrowski Y, Peric M, Koglin S, Ruzicka T, Schauber J. Control of cutaneous antimicrobial peptides by vitamin D3. Arch Dermatol Res 2010;302:401-8.
[Google Scholar]
|
| 9. |
Okasaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by Vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem 1989;264:19076-80.
[Google Scholar]
|
| 10. |
Beilawaska A, Linardic CM, Hannun YA. Modulation of cell growth and differentiation by ceramide. FEBS Lett 1992;307:211-4.
[Google Scholar]
|
| 11. |
Geilen CC, Bektas M, Weider T, Orfanos CR. 1α, 25-dihydroxy vitamin D3 induces sphingomyelin hydrolysis in Ha Ca T cells via tumor necrotic factor α. J Biol Chem 1997;272:8997-9001.
[Google Scholar]
|
| 12. |
Hanada K, Sawamura D, Nakano H, Hashimoto I. Possible role of 1,25-dihydroxy vitamin D3 - induced metallothionein in photoprotection against ultraviolet B injury mouse skin and cultured rat keratinocytes. J Dermatol Sci 1995;9:203-8.
[Google Scholar]
|
| 13. |
Trémezaygues L, Reichrath J. Vitamin D analogues in treatment of psoriasis. Where are we standing and where will we be going. Dermatoendocrinology 2011;3:180-6.
[Google Scholar]
|
| 14. |
Ricketts JR, Rothe JM, Grant-Kels MJ. Nutrition and psoriasis. Clin Dermatol 2010;28:615-26.
[Google Scholar]
|
| 15. |
Van Der Kerkhof PC. Biological activity of vitamin D analogues in the skin, with special reference to antipsoriatic mechanisms. Br J Dermatol 1995;132:675-82.
[Google Scholar]
|
| 16. |
Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, et al. Vitamin D analog differentially control antimicrobial peptide/"alarmin" expression in psoriasis. PLoS One 2009;4:e6340.
[Google Scholar]
|
| 17. |
Holick MF, Reichrath J. Clinical utility of 1, 25-dihydroxy vitamin D3 and its analogs for the treatment of psoriasis. In: Holick MF, editor. Vitamin D. Physiology, Molecular Biologic and Clinical Aspects. Totowa, New York: The Humana Press Inc., 1999. p. 357-73.
[Google Scholar]
|
| 18. |
Park BS, Park JS, Lee DY, Youn JI, Kim IG. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol 1999;112:113-6.
[Google Scholar]
|
| 19. |
Staberg B, Oxholm A, Klemp P, Christiansen C. Abnormal vitamin D metabolism in patients with psoriasis. Acta Derm Venereol 1987;67:65-8.
[Google Scholar]
|
| 20. |
Stewart AF, Battaglini-Sabetta J, Millstone L. Hypocalcemia-induced pustular psoriasis of von Zumbusch. New experience with an old syndrome. Ann Intern Med 1984;100:677-80.
[Google Scholar]
|
| 21. |
Stone OJ. Chloroquine, ground substance, aggravation of psoriasis. Int J Dermatol 1985;24:539.
[Google Scholar]
|
| 22. |
MacLaughlin JA, Gange W, Taylor D, Smith E, Holick MF. Cultured psoriatic fibroblasts from involved and uninvolved sites have partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A 1985;82:5409-12.
[Google Scholar]
|
| 23. |
Morimoto S, Kumahara Y. A patient with psoriasis cured by 1α-hydroxyvitamin D3. Med J Osaka Univ 1985;35:3-4.
[Google Scholar]
|
| 24. |
Kamangar F, Koo J, Heller M, Lee E, Bhutani T. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatolog Treat 2013;24:261-7.
[Google Scholar]
|
| 25. |
Perez A, Raab R, Chen TC, Turner A, Holick MF. Safety and efficacy of oral calcitriol (1,25 dihydroxyvitamin D) for the treatment of psoriasis. Br J Dermatol 1996;134:1070-8.
[Google Scholar]
|
| 26. |
Gaal J, Lakos G, Szodoray P, Kiss J, Horvath I, Horkay E, et al. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow up pilot study. Acta Derm Venereol 2009;89:140-4.
[Google Scholar]
|
| 27. |
Ezquerra GM, Regana MS, Millet PU. Combination of acitretin and oral calcitriol for treatment of plaque-type psoriasis. Acta Derma Venereol 2007;87:449-50.
[Google Scholar]
|
| 28. |
Grace K, Kim DO. The rationale behind topical vitamin D analogues in the treatment of psoriasis. Where does topical calcitriol fit in? J Clin Aesthet Dermatol 2010;3:46-53.
[Google Scholar]
|
| 29. |
Ortonne JP. Calcipotriol in combination with betametasone diproprionate. Nouv Dermatol 1994;13:736-51.
[Google Scholar]
|
| 30. |
Kragballe K. Combination of topical calcipotriol (MC 903) and UVB radiation for psoriasis vulgaris. Dermatologica 1990;181:211-4.
[Google Scholar]
|
| 31. |
Grossman RM, Thivolet J, Claudy A, Souteyrand P, Guilhou JJ, Thomas P, et al. A novel therapeutic approach to psoriasis with combination calcipotriol ointment and very low-dose cyclosporine: A result of a multicenter placebo-controlled study. J Am Acad Dermatol 1994;31:68-74
[Google Scholar]
|
| 32. |
Cambazard, van de Kerkhof PC, Hutchinson PE. The Calcipotriol Study Group Proceedings of the 3 rd International Calcipotriol Symposium. Munich Germany; 1996.
[Google Scholar]
|
| 33. |
van de Kerkhof P. Vitamin D and systemic therapy. Cutis 2002;70:1620.
[Google Scholar]
|
| 34. |
de Jong EM, Mork NJ, Seijger MM, De La Brassine M, Lauharanta J, Jansen CT, et al. The combination of calcipotriol and methotrexate compared with methotrexate and vehicle in psoriasis: Results of a multicentre placebo-controlled randomized trial. Br J Dermatol 2003;148:318-25.
[Google Scholar]
|
| 35. |
Monastirli A, Georgiou S, Pasmatzi E, Sakkis T, Badavanis G, Drainas D, et al. Calcipotriol plus short-contact dithranol: A novel topical combination therapy for chronic plaque psoriasis. Skin Pharmacol Appl Skin Physiol 2002;15:246-51.
[Google Scholar]
|
| 36. |
Campione E, Mazzotta A, Paternò EJ, Diluvio L, Prinz JC, Chimenti S. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol 2009;89:288-91.
[Google Scholar]
|
| 37. |
Solvoll E, Soyland E, Sandstad B, Drevon CA. Dietary habits among patients with atopic dermatitis. Eur J Clin Nutr 2000;54:93-7.
[Google Scholar]
|
| 38. |
Sidbury R, Sullivan AF, Thadhani RI, Camargostr CA Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br J Dermatol 2008;159:245-7.
[Google Scholar]
|
| 39. |
Back O, Blomquist HK, Hernell O, Stenberg B. Does Vitamin D intake during infancy promote the development of atopic allergy? Acta Derma Venereol 2009;89:28-32.
[Google Scholar]
|
| 40. |
Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D dependent mechanism. J Clin Invest 2007;117:803-11.
[Google Scholar]
|
| 41. |
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of vitamin Demediated human antimicrobial response. Science 2006;311:1770-3.
[Google Scholar]
|
| 42. |
Bikle DD, Pillai S, Gee E, Hincenbergo M. Regulation of 1,25-dihydroxy vitamin D production in human keratinocytes by interferon-gamma. Endocrinology 1989;124:655-60.
[Google Scholar]
|
| 43. |
Samochocki Z, Bogaczewicz J, Jeziorkowska R, Sysa-Jędrzejowska A, Glińska O, Karczmarewicz E, et al. Vitamin D effects in atopic dermatitis. J Am Acad Dermatol 2013;69:238-44.
[Google Scholar]
|
| 44. |
Ong PY, Ohtahe T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151-60.
[Google Scholar]
|
| 45. |
Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol 2006;117:836-41.
[Google Scholar]
|
| 46. |
Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 2008;122:829-31.
[Google Scholar]
|
| 47. |
Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol 2011;164:1078-82.
[Google Scholar]
|
| 48. |
Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, et al. Vitamin D supplementation in the treatment of atopic dermatitis: A clinical study. J Drugs Dermatol 2012;11:327-30.
[Google Scholar]
|
| 49. |
Le Poole IC, Wankowicz-Kalinska A, van den Wijngaard RM, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Invest Dermatol Symp Proc 2004;9:68-72.
[Google Scholar]
|
| 50. |
Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol 2008;4:404-12.
[Google Scholar]
|
| 51. |
Silverberg JI, Silverberg AI, Malka E, Silverberg NB. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol 2010;62:937-41.
[Google Scholar]
|
| 52. |
Li K, Shi Q, Yang L, Li X, Liu L, Wang L, et al. The association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with generalized vitiligo. Br J Dermatol 2012;167:815-21.
[Google Scholar]
|
| 53. |
Parsad D, Saini R, Verma N. Combination of PUVAsol and topical calcipotriol in vitiligo. Dermatology 1998;197:167-70.
[Google Scholar]
|
| 54. |
AlGhamdi K, Noura A, Moussa N. The role of vitamin D in melanogenesis with an emphasis on vitiligo. Indian J Dermatol Venereol Leprol 2013;79:750-8.
[Google Scholar]
|
| 55. |
Parsad D, Kanwar AJ. Topical vitamin D analogues in the treatment of vitiligo. Pigment Cell Melanoma Res 2009;22:487-8.
[Google Scholar]
|
| 56. |
Xing C, Xu A. The effect of combined calcipotriol and betamethasone dipropionate ointment in the treatment of vitiligo: An open, uncontrolled trial. J Drugs Dermatol 2012;11:e52-4.
[Google Scholar]
|
| 57. |
Rodríguez-Martín M, García Bustínduy M, Sáez Rodríguez M, Noda Cabrera A. Randomized, double-blind clinical trial to evaluate the efficacy of topical tacalcitol and sunlight exposure in the treatment of adult nonsegmental vitiligo. Br J Dermatol 2009;160:409-14.
[Google Scholar]
|
| 58. |
Birlea SA, Costin GE, Norri DA. New insights on therapy with vitamin D analogs targeting the intracellular pathways that control repigmentation in human vitiligo. Med Res Rev 2009;29:514-46.
[Google Scholar]
|
| 59. |
Goldinger SM, Dummer R, Schmid P, Burg G, Seifert B, Läuchli S. Combination of 308-nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J Eur Acad Dermatol Venereol 2007;21:504-8.
[Google Scholar]
|
| 60. |
Oh SH, Kim T, Jee H, Do JE, Lee JH. Combination treatment of non-segmental vitiligo with a 308-nm xenon chloride excimer laser and topical high-concentration tacalcitol: A prospective, single-blinded, paired, comparative study. J Am Acad Dermatol 2011;65:428-30.
[Google Scholar]
|
| 61. |
Finamor DC, Sinigaglia-Coimbra R, Neves LC, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol 2013;5:222-34.
[Google Scholar]
|
| 62. |
Markowitz ME. Rickets associated with hypophosphatemia. In: Pediatric Endocrinology: Mechanisms, Manifestations and Management. In: Pescovitz OH, Eugster EA, editors. Philadelphia: Lippincott Williams and Wilkins; 2004. p. 653-67.
[Google Scholar]
|
| 63. |
Griffiths WA, Judge MR, Leigh IM. Disorders of keratinization. In: Wilkinson R, editor. Ebling Textbook of Dermatology. 6 th ed. Oxford: Blackwell Science; 1998. p. 1483-8.
[Google Scholar]
|
| 64. |
Ingen-Housz-Oro S, Boudou P, Bergot C, Ibrahim F, Souberbielle JC, Dubertret L, et al. Evidence of a marked 25-hydroxyvitamin D deficiency in patients with congenital ichthyosis. J Eur Acad Dermatol Venereol 2006;20:947-52.
[Google Scholar]
|
| 65. |
Sethuraman G, Khaitan BK, Dash SS, Chandramohan K, Sharma VK, Kabra M, et al. Ichthyosiform erythroderma with rickets: Report of five cases. Br J Dermatol 2008;158:603-6.
[Google Scholar]
|
| 66. |
Milstone LM, Ellison AF, Insogna KL. Serum parathyroid hormone level is elevated in some patients with disorders of keratinization. Arch Dermatol 1992;128:926-30.
[Google Scholar]
|
| 67. |
Milstone LM, Bale SJ, Insogna KL. Secondary hyperparathyroidism in patients with ichthyosis is not caused by vitamin D deficiency or ingestion of retinoids. Arch Dermatol 1993;129:648.
[Google Scholar]
|
| 68. |
Thacher TD, Fischer PR, Pettifor JM, Darmstadt GL. Nutritional rickets in ichthyosis and response to calcipotriene. Pediatrics 2004;114:e119-23.
[Google Scholar]
|
| 69. |
Okano M, Kitano Y, Yoshikawa K. A trial of oral 1 alpha-hydroxyvitamin D3 for ichthyosis. Dermatologica 1988;177:23.
[Google Scholar]
|
| 70. |
el-Khateeb EA. Bullous congenital ichthyosiform erythroderma associated with hypocalcemic vitamin D-resistant rickets. Pediatr Dermatol 2008;25:279-82.
[Google Scholar]
|
| 71. |
Kothari D, Doshi B, Garg G, Khopkar US. Ichthyosis associated with rickets in two Indian children. Indian J Dermatol 2013;58:244.
[Google Scholar]
|
| 72. |
Kumar V, Kalra S, Mutreja D, Arya A. Rickets associated with icthyosis. Pediatr Int Child Health 2012;32:119-20.
[Google Scholar]
|
| 73. |
Chouhan K, Sethuram G, Gupta N, Sharma VK, Kabra M, Khaitan BK, et al. Vitamin D deficiency and rickets in children and adolescents with icthyosiform erythroderma in type IV and V skin. Br J Dermatol 2012;166:608-15.
[Google Scholar]
|
| 74. |
Deka N, Sarma D, Saikia UK. Lamellar icthyosis with genu valgum: Unfolding the link. BMJ Case Rep 2012;2012.
[Google Scholar]
|
| 75. |
Vupputuri MR, Goswami R, Gupta N, Ray D, Tandon N, Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am J Clin Nutr 2006;83:1411-9.
[Google Scholar]
|
| 76. |
Studzinski GP, Moore DC. Sunlight- can it prevent as well as cause cancer? Cancer Res 1995;55:4014-22.
[Google Scholar]
|
| 77. |
Langberg M, Rotem C, Fenig E, Koren R, Ravid A. Vitamin D protects keratinocytes from deleterious effects of ionizing radiation. Br J Dermatol 2009;160:151-61.
[Google Scholar]
|
| 78. |
Nürnberg B, Gräber S, Gärtner B, Geisel J, Pföhler C, Schadendorf D, et al. Reduced serum 25- hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res 2009;29:3669-74.
[Google Scholar]
|
| 79. |
Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer- ready for prime time? N Engl J Med 2011;364:1385-7.
[Google Scholar]
|
| 80. |
Gorham ED, Mohr SB, Garland CF, Chaplin G, Garland FC. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol 2007;17:956-63.
[Google Scholar]
|
| 81. |
Asgari MM, Maruti SS, Kushi LH, White E. A cohort study of vitamin D Intake and melanoma risk. J Invest Dermatol 2009;129:1675-80.
[Google Scholar]
|
| 82. |
Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer 2009;45:3271-81.
[Google Scholar]
|
| 83. |
Zhang GY, Cheng T, Luan Q, Liao T, Nie CL, Zheng X, et al. Vitamin D: A novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol 2011;164:729-37.
[Google Scholar]
|
| 84. |
Boelsma E, Pavel S, Ponee M. Effects of calcitriol on fibroblasts derived from skin of scleroderma patients. Dermatology 1995;191:226-33.
[Google Scholar]
|
| 85. |
Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Clinical significance of serum level of soluble interleukin-2 receptor in patients with localized scleroderma. Br J Dermatol 1996;134:843-7.
[Google Scholar]
|
| 86. |
Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 1984;133:1748-54.
[Google Scholar]
|
| 87. |
Tay YK. Topical calcipotriol ointment in the treatment of morphea. J Dermatolog Treat 2003;14:219-21.
[Google Scholar]
|
| 88. |
Hulshof MM, Pavel S, Breedveld FC, Dijkmans BA, Vermeer BJ. Oral calcitriol as a new therapeutic for generalized morphea. Arch Dermatol 1994;130:1290-3.
[Google Scholar]
|
| 89. |
Kreuter A, Gambichler T, Sauermannn K, Jansen T, Altmeyer P, Hoffmann K. Extragenital lichen sclerosus successfully treated with topical calcipotriol: Evaluation by in vivo confocal laser scanning microscopy. Br J Dermatol 2002;146:332-3.
[Google Scholar]
|
| 90. |
Peacocke M, Djurkinak E, Thys-Jacobs S. Treatment of desquamative inflammatory vaginitis with vitamin D: A case report. Cutis 2008;81:75-8.
[Google Scholar]
|
| 91. |
Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr 2009;139:1157-61.
[Google Scholar]
|
| 92. |
Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009;6:231-43.
[Google Scholar]
|
| 93. |
Dowling GB, Gauvain S, Macrae DE. Vitamin D in treatment of cutaneous tuberculosis. Br Med J 1948;1:430-5.
[Google Scholar]
|
| 94. |
Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int J Epidemiol 2008;37:113-9.
[Google Scholar]
|
| 95. |
Kriegel MA, Manson JE, Costenbader KH. Does vitamin D affect risk of developing autoimmune disease?: A systematic review. Semin Arthritis Rheum 2011;40:512-31.
[Google Scholar]
|
| 96. |
Lu′o′ng Kv, Nguyên LT. The roles of vitamin D in seborrhoeic keratosis: Possible genetic and cellular signalling mechanisms. Int J Cosmet Sci 2013;35:525-31.
[Google Scholar]
|
| 97. |
Ekiz O, Balta I, Sen BB, Dikilitaº MC, Ozuğuz P, Rifaioğlu EN. Vitamin D status in patients with rosacea. Cutan Ocul Toxicol 2014;33:60-2.
[Google Scholar]
|
| 98. |
Reichrath J. Unravelling of hidden secrets: The role of vitamin D in skin aging. Dermatoendocrinol 2012;4:241-4.
[Google Scholar]
|
| 99. |
Rasheed H, Mahgoub D, Hegazy R, El-Komy M, Abdel Hay R, Hamid MA, et al. Serum ferritin and vitamin D in female hair loss: Do they play a role? Skin Pharmacol Physiol 2013;26:101-7.
[Google Scholar]
|
| 100. |
Burkiewicz CJ, Guadagnin FA, Skare TL, do Nascimento MM, Servin SC, de Souza GD. Vitamin D and skin repair: A prospective, double-blind and placebo controlled study in the healing of leg ulcers. Rev Col Bras Cir 2012;39:401-7.
[Google Scholar]
|
| 101. |
Agak GW, Qin M, Nobe J, Kim MH, Krutzik SR, Tristan GR, et al. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol 2014;134:366-73.
[Google Scholar]
|
| 102. |
Russell M. Assessing the relationship between vitamin D3 and stratum corneum hydration for the treatment of xerotic skin. Nutrients 2012;4:1213-8.
[Google Scholar]
|
| 103. |
Can M, Gunes M, Haliloglu OA, Haklar G, Inanç N, Yavuz DG, et al. Effect of vitamin D deficiency and replacement on endothelial functions in Behçet′s disease. Clin Exp Rheumatol 2012;30:S57-61.
[Google Scholar]
|
| 104. |
Marzano AV, Trevisan V, Eller-Vainicher C, Cairoli E, Marchese L, Morelli V, et al. Evidence for vitamin D deficiency and increased prevalence of fractures in autoimmune bullous skin diseases. Br J Dermatol 2012;167:688-91.
[Google Scholar]
|
| 105. |
Pal L, Berry A, Coraluzzi L, Kustan E, Danton C, Shaw J, et al. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol 2012;28:965-8.
[Google Scholar]
|
| 106. |
Krul-Poel YH, Snackey C, Louwers Y, Lips P, Lambalk CB, Laven JS, et al. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome: A systematic review. Eur J Endocrinol 2013;169:853-65.
[Google Scholar]
|
| 107. |
Harinarayan CV, Joshi SR. Vitamin D status in India-Its implications and remedial measures. J Assoc Physicians India 2009;57:40-8.
[Google Scholar]
|
| 108. |
Marwaha RK, Sripathy G. Vitamin D and bone mineral density of healthy school children in northern India. Indian J Med Res 2008;127:239-44.
[Google Scholar]
|
| 109. |
Londhey V. Vitamin D deficiency: Indian scenario. J Assoc Physicians India 2011;59:695-6.
[Google Scholar]
|
| 110. |
Harinarayan CV, Holick MF, Prasad UV, Vani PS, Himabindu G. Vitamin D status and sun exposure in India. Dermatoendocrinol 2013;5:130-41.
[Google Scholar]
|
| 111. |
Report of Joint FAO/WHO Expert Consultation on vitamin and mineral requirement in human nutrition: Bangkok 1998. Second Edition FAO Rome, 2004. Available from: http://www. whqlibdoc.who.int/publications/2004/9241546123.pdf. Vitamin and mineral requirements in human nutrition: Report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21-30 September 1998.
[Google Scholar]
|
Fulltext Views
38,805
PDF downloads
5,185





