Translate this page into:
Vitiligo pathogenesis is interlinked with pigment homeostasis: A new concept
Correspondence Address:
Ratnam Attili
Visakha Institute of Skin and Allergy, Marripalem, Visakhapatnam - 530 018, Andhra Pradesh
India
| How to cite this article: Attili R, Attili SK. Vitiligo pathogenesis is interlinked with pigment homeostasis: A new concept. Indian J Dermatol Venereol Leprol 2017;83:630-634 |
Global research to resolve the mystery of vitiligo has been progressing in several directions, resulting in divergent data.[1] Such jigsaw pieces when integrated in to a comprehensive overview suggest that vitiligo is an outcome of a localized or generalized intrinsic malady of melanocytes that may evoke immune reactivity and thereby suffer destruction.[2] As melanocytes belong to the long arms of a centrally operating pigment homeostatic system, any pathology that involves melanocytes has to be considered in the larger context of their origin, survival, and replacement. In the light of this new perspective, current concepts are reviewed and course corrections suggested for future research.
Diagnosis and Clinical Types
Although many clinical patterns of vitiligo have been identified, consensus is achieved only in cases of generalized vitiligo with lesions in bilateral symmetric patterns either acral or trunk dominant; and segmental vitiligo, when lesions are confined to one small or large anatomical segment. Single lesions with well-defined margins usually associated with leukotrichia are considered as focal vitiligo.[2] The diagnosis becomes contentious with other patterns, which can be difficult to differentiate from post inflammatory depigmentation seen in several diseases.[3]
The current practice is to classify vitiligo as segmental, non-segmental, or mixed types.[4],[5],[6] However, in the light of our recent data indicating anatomical segmentations in all forms of vitiligo, it would be more appropriate to consider vitiligo as mono segmented, bilateral multi segmented and a universal disease.[2]
Pathogenesis
Anatomical segmentation when considered along with melanocytorrhagy (intrinsic anchoring and survival problems of melanocytes)[7],[8],[9],[10],[11],[12] suggests that the clinical expression of vitiligo is a mosaic developmental malady. Many extrinsic and intrinsic adverse factors may cut short the life span of such abnormal melanocytes necessitating their rapid turnover, which may in turn, enhance the risk of exposing immunogenic melanocyte-differentiating antigens.[13] The clinical expression of vitiligo depends upon the number of defective mosaics and the nature/severity of immune reaction-localized or disseminated.[14],[15],[16],[17] This hypothesis [Figure - 1] is supported by the association of vitiligo with halo nevi and melanoma immunotherapy.[18],[19],[20],[21],[22],[23],[24] Vitiligo and nevi, both considered as mosaic malformations (structural and/functional) of melanocytes,[25],[2] may evoke similar immune reactions against melanocyte antigens, albeit with different outcomes: depigmentation in vitiligo or resorption in the case of pigmented nevi. Interestingly, both these phenomena are seen together in halo nevi. The unique circular segmentations akin to the small circular anatomical segments seen in vitiligo [Figure - 2], indicate that the pigmented nevus along with its halo area may represent a combined mosaic defect of melanocytes (structural and/or functional), brought to clinical notice by the immune reaction against shared antigens. Ultimately, normal pigmented skin replaces the halo as well as the nevus; a unique phenomenon where defective mosaics are repaired by pigment homeostatic mechanisms. In some instances, common pigmented nevi may de novo provoke limited immune reactions resulting in the resorption of the nevi. We have also observed for the first time, a giant congenital nevus provoking generalized vitiligo in a middle-aged patient [Figure - 3]. This was not a mere association, as the depigmentation initially occurred only along the nevus (which was distributed along Blaschko's lines), but subsequently disseminated to normal pigmented skin in other areas. This suggests that immune reactions can arise anytime and become proactive beyond the nevus. Biopsies taken from the pigmented nevus and the adjacent depigmented skin demonstrated destruction of nevus nests due to lymphocytic infiltration around basal melanocytes, as seen in vitiligo. On the other hand, we have also observed halo nevi appearing along with or consequent to disseminated vitiligo, some of which failed to regain pigment even after the nevus disappeared. This may be because the immune reaction in generalized vitiligo not only destroys the pigmented nevus, but at the same time opposes the melanocyte recovery by normal pigment homeostasis. It would be interesting to study the fate of common pigmented nevi in patients suffering from universal vitiligo.
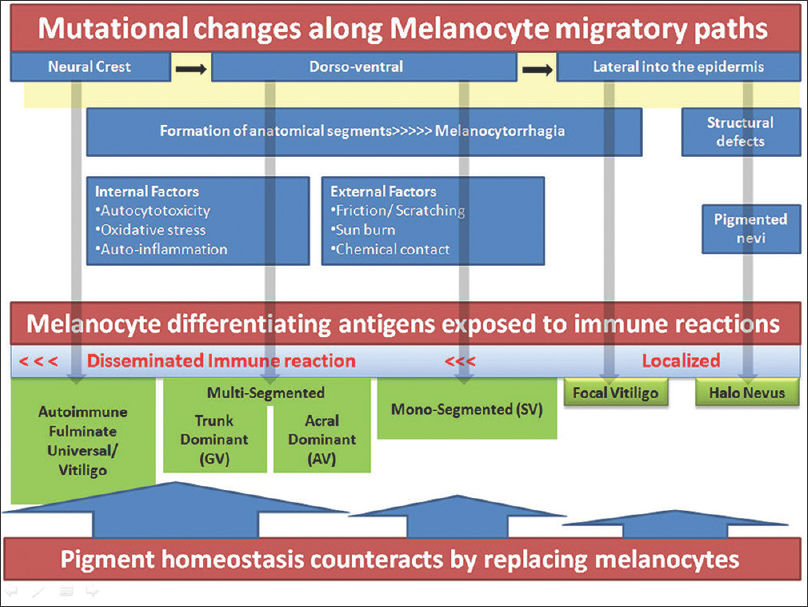 |
| Figure 1: Schematic presentation of vitiligo pathogenesis. Top line across shows melanocyte migrations from the neural crest through many anatomical segments to the skin. Mutations that may occur along the long paths result in functional or nevoid defects of melanocytes. Generalized symmetric disease results when mutations occur before bilateral development, mono or bilateral segmental disease when they occur distally at the formation of various anatomical parts and focal lesions when the terminal paths are affected. Defective melanocytes (functional or nevoid accumulations) may attract immune reactions and destruction. Pigment homeostasis counteracts by activating fresh migrations from stem cell niche persisting along the migratory paths |
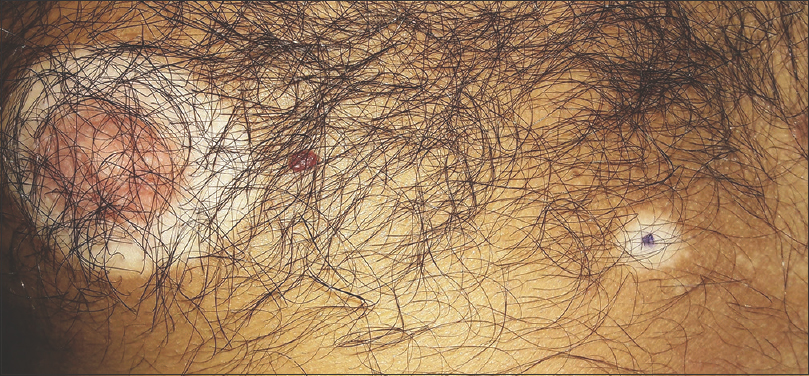 |
| Figure 2: Similar circular segmentations of vitiligo and halo nevus |
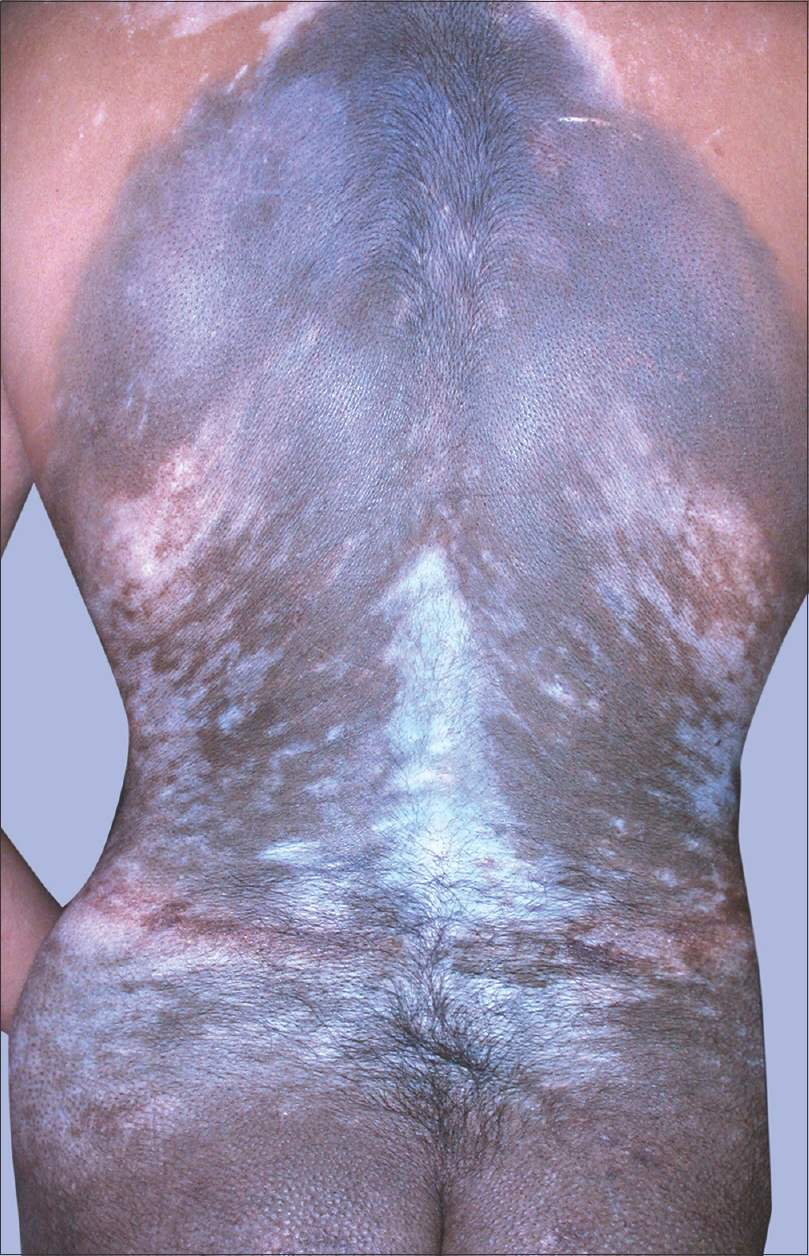 |
| Figure 3: Generalized vitiligo developing in a patient with congenital giant pigmented nevus |
Insidious and fulminate types of evolution
Immune reactions in vitiligo can be localized or generalized, insidious, or fulminate. The most commonly seen acral disease with bilateral segmented lesions has an insidious onset with limited involvement. The lesions may resolve spontaneously or while remaining stable for long periods [Figure - 4], progress incrementally towards the trunk. Acral disease usually responds poorly to treatment.[26] A possible explanation could be that the longer migratory paths not only increase the risk of melanocytorrhagy, but also hinder pigment recovery.[2] Failure to cope with the localized but persisting immune reaction which increases the demand for melanocyte replacements, may result in centripetal extension of depigmentation. In contrast, fulminate generalized disease may arise from a de novo generalized autoimmune reaction, overwhelming the pigment homeostatic mechanism. Future studies need to test different hypothesis offered in these two distinct forms of vitiligo to explain the differences in their evolution and behaviour.
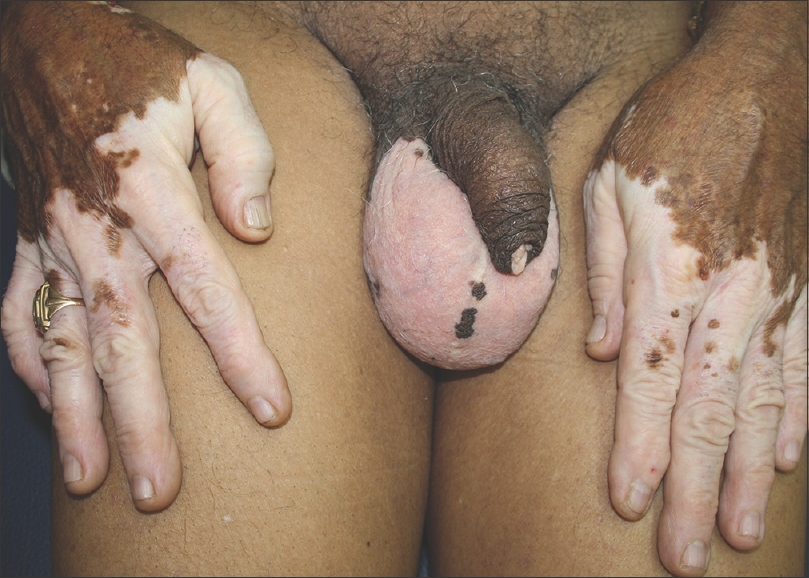 |
| Figure 4: Acral vitiligo with stable lesions for over 50 years: Genital segmentation |
Pigment homeostasis
Steady streaming of melanocyte precursors along innumerable migratory pathways, arising from stem cell pools is probably a life time physiological feature to replace affected melanocytes.[2] Such a homeostatic mechanism becomes seriously compromised when melanocyte differentiating antigens become the target of immune reactions, thus blocking the final conversion of melanoblast to melanocyte. This hypothesis explains discordant reports- presence or absence of functional melanocytes in vitiligo,[27],[28] as the dynamic balance between the two opposing forces (immune reaction and melanocyte migration along bilateral symmetrical paths) may shift one way or the other at different periods. The initial pigment loss from acral areas and recovery in the opposite direction (as mirror image progression and regression), adds support to such a hypothesis. Pigment loss and recovery patterns in vitiligo indicate persistence of stem cell niches at all the anatomical junctional areas through which the melanocytes migrate. Stem cells in the follicular bulge are known to control the follicular-epidermal paths and when affected, result in punctate depigmentation in progressive disease [Figure - 5] and punctate repigmentation in the regressive phase [Figure - 6]. Vitiligo with the exception of fulminate disease, does not involve all the anatomical segments at the same time. Therefore, progressive, stable, and regressive lesions can be concurrently seen in different anatomical areas. This common observation indicates presence of independent stem cell niches within each anatomical segment. On the other hand, bilateral anatomical segments showing progression and regression in perfect unison suggest presence of stem cell pools at the dorsal midline of the body. This is exemplified in some cases of long standing universal vitiligo with pigment recovering in bilateral symmetrical waves [Figure - 7]. This gives the impression of fresh melanocyte migrations gaining over quiescent immune reactivity. Such migrations appear to override the follicular stem cell niche which usually induces spotty repigmentation [Figure - 6].
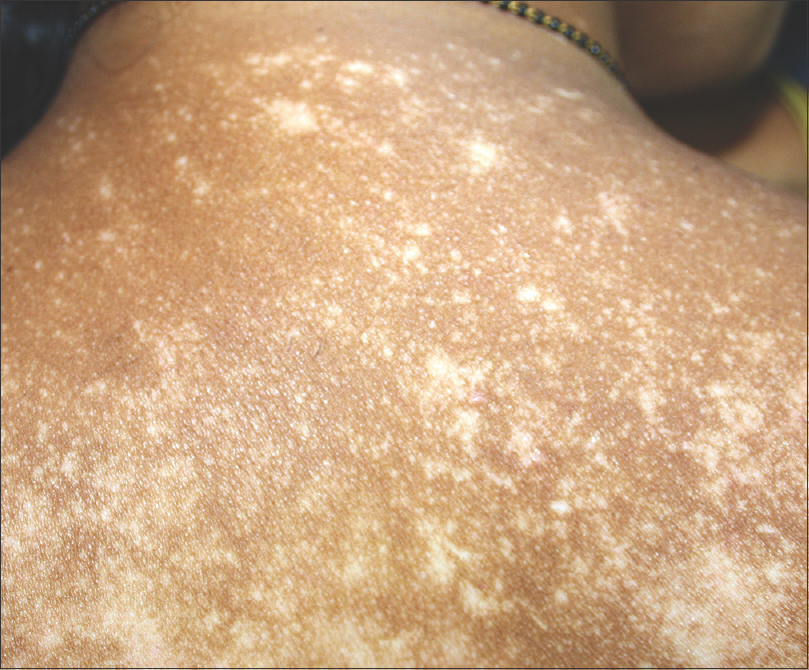 |
| Figure 5: Punctate follicular depigmentation in the evolution of vitiligo |
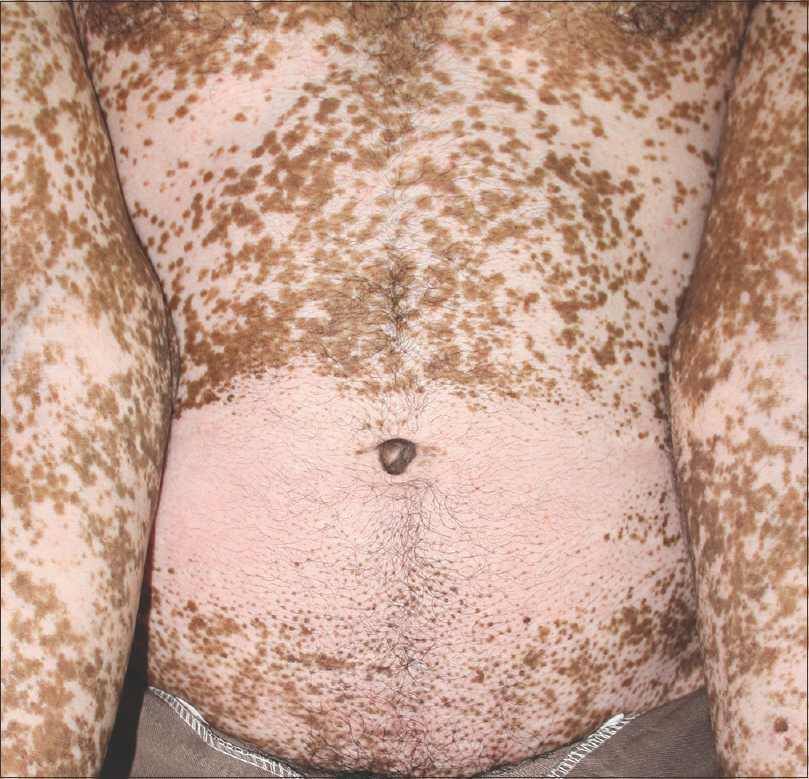 |
| Figure 6: Punctate follicular repigmentation in regressive phase |
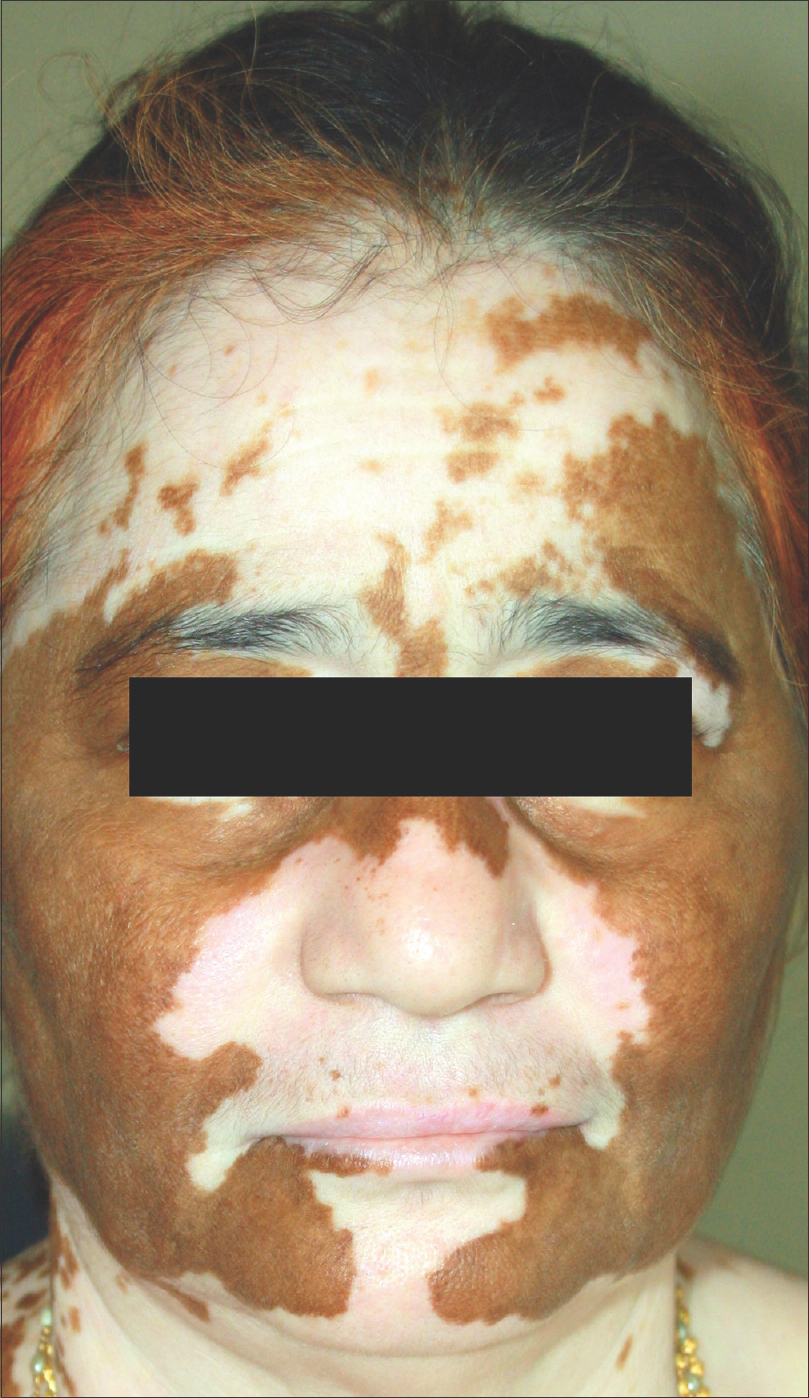 |
| Figure 7: Spontaneous repigmentation spreading along bilateral symmetrical pathways in universal vitiligo cases |
Several studies elucidated the role of immune reactions in vitiligo, but not much is known about pigment homeostatic mechanisms; in particular, information on the natural life span and replacement of melanocytes in the epidermis. There are reports of keratinocytes playing a predominant role in the homeostatic regulation of melanocytes within the epidermis and they might also be implicated in the regulation of melanocyte stem cells. Additionally, dermal fibroblasts are also believed to be involved in melanocyte regulation. How melanocyte stem cells are regulated by cells in other lineages is unclear, with the molecular mechanisms remaining elusive.[29]
Although clinical observations indicate presence of melanocyte stem cell niches along the migratory paths at multiple levels, locations other than the follicular bulge are yet to be explored. The role of the human pituitary gland and alpha-MSH in pigment regulation is uncertain.[30],[31],[32],[33] Melanocyte precursors have been reported in the follicular bulge, infundibulum, and inter follicular epidermis in cases of vitiligo treated with narrow band ultraviolet light, indicating activated peripheral stem cell migration.[34] At a higher level, bolstering of Wnt signalling which can induce common origin cells to shift from the neural lines to the melanocytic lineage;[35],[36],[37] and c-kit receptors that are reported to enhance melanocyte migrations to the skin,[38] may offer new avenues for treatment.
Conclusions
Depigmentation in vitiligo is proposed as an outcome of a dynamic tug of war between the immune system (destroying developmentally defective melanocytes) and the pigment homeostatic mechanism (which is counteracting by providing melanocyte replacements). This concept depends upon the assumption of anatomical mosaic defects (melanocytorrhagy), which need to be investigated in all forms of vitiligo. The suggested role of pigment homeostasis based upon clinical evidence, can be considered speculative. Nevertheless, it should stimulate researchers to investigate and identify stem cell locations and their biological control mechanisms, beyond the follicular units.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lotti T, Hautmann G, Hercogova J, editors. Vitiligo: Disease or symptom? From the confusion of the past to current doubts. Vitiligo: Problems and Solutions. New York: Marcel Dekker Inc.; 2004. p. 1-15.
[Google Scholar]
|
| 2. |
Attili VR, Attili SK. Anatomical segmentations in all forms of vitiligo: A new dimension to the etiopathogenesis. Indian J Dermatol Venereol Leprol 2016;82:379-88.
[Google Scholar]
|
| 3. |
Liao W, Nordlund JJ. Differential diagnosis in vitiligo. In: Lotti T, Hautmann G, Hercogova J, editors. Vitiligo: Problems and Solutions. New York: Marcel Dekker Inc.; 2004. p. 207-25.
[Google Scholar]
|
| 4. |
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: The vitiligo global issues consensus conference. Pigment Cell Melanoma Res 2012;25:E1-13.
[Google Scholar]
|
| 5. |
Ezzedine K, Gauthier Y, Léauté-Labrèze C, Marquez S, Bouchtnei S, Jouary T, et al. Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): A retrospective case series of 19 patients. J Am Acad Dermatol 2011;65:965-71.
[Google Scholar]
|
| 6. |
Mulekar SV, Al Issa A, Asaad M, Ghwish B, Al Eisa A. Mixed vitiligo. J Cutan Med Surg 2006;10:104-7.
[Google Scholar]
|
| 7. |
Cario-André M, Pain C, Gauthier Y, Taïeb A. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res 2007;20:385-93.
[Google Scholar]
|
| 8. |
Namazi MR. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: Can they be interconnected? Pigment Cell Res 2007;20:360-3.
[Google Scholar]
|
| 9. |
Gauthier Y, Cario Andre M, Taïeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 2003;16:322-32.
[Google Scholar]
|
| 10. |
Kumar R, Parsad D. Melanocytorrhagy and apoptosis in vitiligo: Connecting jigsaw pieces. Indian J Dermatol Venereol Leprol 2012;78:19-23.
[Google Scholar]
|
| 11. |
Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol 2011;164:187-91.
[Google Scholar]
|
| 12. |
Wagner RY, Luciani F, Cario-André M, Rubod A, Petit V, Benzekri L, et al. Altered e-cadherin levels and distribution in melanocytes precede clinical manifestations of vitiligo. J Invest Dermatol 2015;135:1810-9.
[Google Scholar]
|
| 13. |
Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol 2003;121:550-6.
[Google Scholar]
|
| 14. |
Attili VR, Attili SK. Lichenoid inflammation in vitiligo – A clinical and histopathologic review of 210 cases. Int J Dermatol 2008;47:663-9.
[Google Scholar]
|
| 15. |
Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol 1996;148:1219-28.
[Google Scholar]
|
| 16. |
Le Poole IC, Wañkowicz-Kaliñska A, van den Wijngaard RM, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Investig Dermatol Symp Proc 2004;9:68-72.
[Google Scholar]
|
| 17. |
Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: A convergence theory. Exp Dermatol 1993;2:145-53.
[Google Scholar]
|
| 18. |
Naveh HP, Rao UN, Butterfield LH. Melanoma-associated leukoderma-Immunology in black and white? Pigment Cell Melanoma Res 2013;26:796-804.
[Google Scholar]
|
| 19. |
Hale EK, Konstadt JW. Melanoma-associated leukoderma. Dermatol Online J 2003;9:20.
[Google Scholar]
|
| 20. |
Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J Clin Oncol 2015;33:773-81.
[Google Scholar]
|
| 21. |
van Geel N, Vandenhaute S, Speeckaert R, Brochez L, Mollet I, De Cooman L, et al. Prognostic value and clinical significance of halo naevi regarding vitiligo. Br J Dermatol 2011;164:743-9.
[Google Scholar]
|
| 22. |
van Geel N, Speeckaert R, Lambert J, Mollet I, De Keyser S, De Schepper S, et al. Halo naevi with associated vitiligo-like depigmentations: Pathogenetic hypothesis. J Eur Acad Dermatol Venereol 2012;26:755-61.
[Google Scholar]
|
| 23. |
Silveira ML, Ferreira FR, Alvarenga ML, Mandelbaum SH. Association of giant congenital melanocytic nevus, halo nevus and vitiligo in a 75-year-old patient. An Bras Dermatol 2012;87:288-91.
[Google Scholar]
|
| 24. |
Ezzedine K, Diallo A, Léauté-Labrèze C, Seneschal J, Mossalayi D, AlGhamdi K, et al. Halo nevi association in nonsegmental vitiligo affects age at onset and depigmentation pattern. Arch Dermatol 2012;148:497-502.
[Google Scholar]
|
| 25. |
Kouzak SS, Mendes MS, Costa IM. Cutaneous mosaicisms: Concepts, patterns and classifications. An Bras Dermatol 2013;88:507-17.
[Google Scholar]
|
| 26. |
Attili VR, Attili SK. Acral vitiligo and lichen sclerosus-Association or a distinct pattern? A clinical and histopathological Review of 15 Cases. Indian J Dermatol 2015;60:519.
[Google Scholar]
|
| 27. |
Tobin DJ, Swanson NN, Pittelkow MR, Peters EM, Schallreuter KU. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol 2000;191:407-16.
[Google Scholar]
|
| 28. |
Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: An immunohistochemical investigation. J Invest Dermatol 1993;100:816-22.
[Google Scholar]
|
| 29. |
Lee AY. Role of keratinocytes in the development of vitiligo. Ann Dermatol 2012;24:115-25.
[Google Scholar]
|
| 30. |
Thody AJ, Graham A. Does alpha-MSH have a role in regulating skin pigmentation in humans? Pigment Cell Res 1998;11:265-74.
[Google Scholar]
|
| 31. |
Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol 2013;88:76-83.
[Google Scholar]
|
| 32. |
Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem 2002;50:125-33.
[Google Scholar]
|
| 33. |
Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res 1997;10:288-97.
[Google Scholar]
|
| 34. |
Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, et al. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol 2015;135:2068-76.
[Google Scholar]
|
| 35. |
Harris JE. Melanocyte regeneration in vitiligo requires WNT beneath their wings. J Invest Dermatol 2015;135:2921-3.
[Google Scholar]
|
| 36. |
Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci U S A 2000;97:10050-5.
[Google Scholar]
|
| 37. |
Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998;396:370-3.
[Google Scholar]
|
| 38. |
Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol 2006;126:1102-10.
[Google Scholar]
|
Fulltext Views
3,404
PDF downloads
1,617





