Translate this page into:
Viva questions from the IJDVL
2 Department of Dermatology, K J Somaiya Medical College and Research Centre, Mumbai, Maharashtra, India
Correspondence Address:
Vishalakshi Viswanath
Department of Dermatology, Rajiv Gandhi Medical College, Thane, Maharashtra
India
| How to cite this article: Viswanath V, Vasani R. Viva questions from the IJDVL. Indian J Dermatol Venereol Leprol 2016;82:240-245 |
Topical Corticosteroids
What is the structure of the steroid molecule?
All steroids are composed of cyclopentano-perhydro-phenanthrene ring. It is composed of three 3C rings and one 5C ring.
To increase the potency of topical steroid, the structural modifications done are:
- C-6a or C-9a fluorination
- C-21 halogenation
- C-17, C-21 esterification
- Double bond between C-1 and C-2.
Where would one preferentially use low-potent steroids?
In conditions and areas where higher absorption of topical steroids can occur, it is preferable to use low-potent steroids.
- Absorption of topical steroid varies according to the anatomical location. Absorption from the forearm is poor (1%), it is around 4% from the scalp whereas that from the scrotum is around 35% of the applied drug. The absorption of topical steroid from the eyelid is 300 times greater than from plantar skin. Intertriginous areas are particularly susceptible to higher absorption owing to thinner skin, increased moisture, elevated temperature and partial occlusion provided by the location
- Children are more susceptible to increased absorption of the topically applied steroid and subsequent side effects on account of increased ratio of total body surface area to body weight (2.5-3-fold of adults)
- Larger body surface area involvement in dermatoses that impair barrier function such as atopic dermatitis allows increased absorption of the topical steroids with subsequent side effects.
Hence, in the above situations, a low-potency steroid application is preferred. In addition, use of oral therapy and early use of steroid-sparing agents, wherever applicable, helps to avoid the side effects of topical steroids.
What will be the topical steroid of choice in [Figure - 1]? Give reasons for choosing the particular topical steroid?
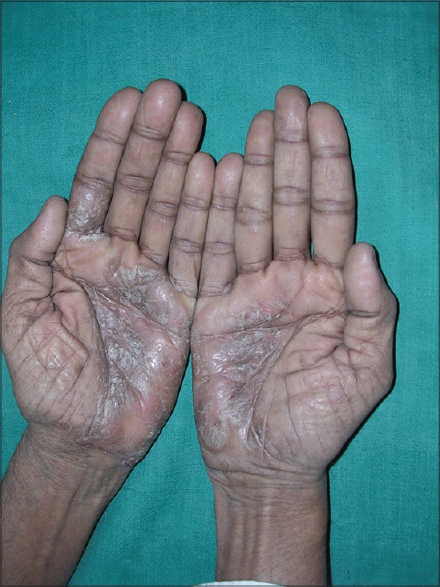 |
| Figure 1: Enumerate the topical steroid of choice for this condition |
Considering a diagnosis of palmar psoriasis, a class I superpotent steroid-like clobetasol propionate 0.05% in combination with 3% salicylic acid in an ointment base will be preferred to be applied 2 times a day only on the affected area.
The reasons for this choice are the chronic hyperkeratotic nature of the lesion. Poor penetration of the topical application may be expected due to a thickened stratum corneum. In addition, a greater frequency of application is required since the product is easily removed from the hands during normal activities. An ointment base is preferred since it enhances the penetration of the drug by its occlusive effect and increased hydration of the stratum corneum. Prehydration of the skin after soaking in water with occlusion of the affected area by using gloves will increase penetration and may hasten improvement.
How can one optimize the use of topical steroids?
- Start with an appropriately potent compound to achieve rapid disease control
- Continue with a less potent preparation after sufficient response
- Reduce frequency of application (alternate-day therapy; weekend use)
- Continue daily application with the weakest effective preparation
- ′′Taper off" treatment upon complete healing
- Take particular care in treating children and the elderly, especially at certain locations (e.g., scrotum, face, flexures and area around eyes).
Enumerate the side effects of topical corticosteroids
The side effects of topical corticosteroids are as enumerated in Box 1.

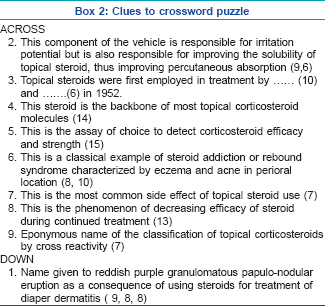
Crossword puzzle
Fill in the crossword puzzle in [Figure - 2] using the clues provided below in Box 2. Answers to the crossword puzzle are provided in [Figure - 3] on page 245.
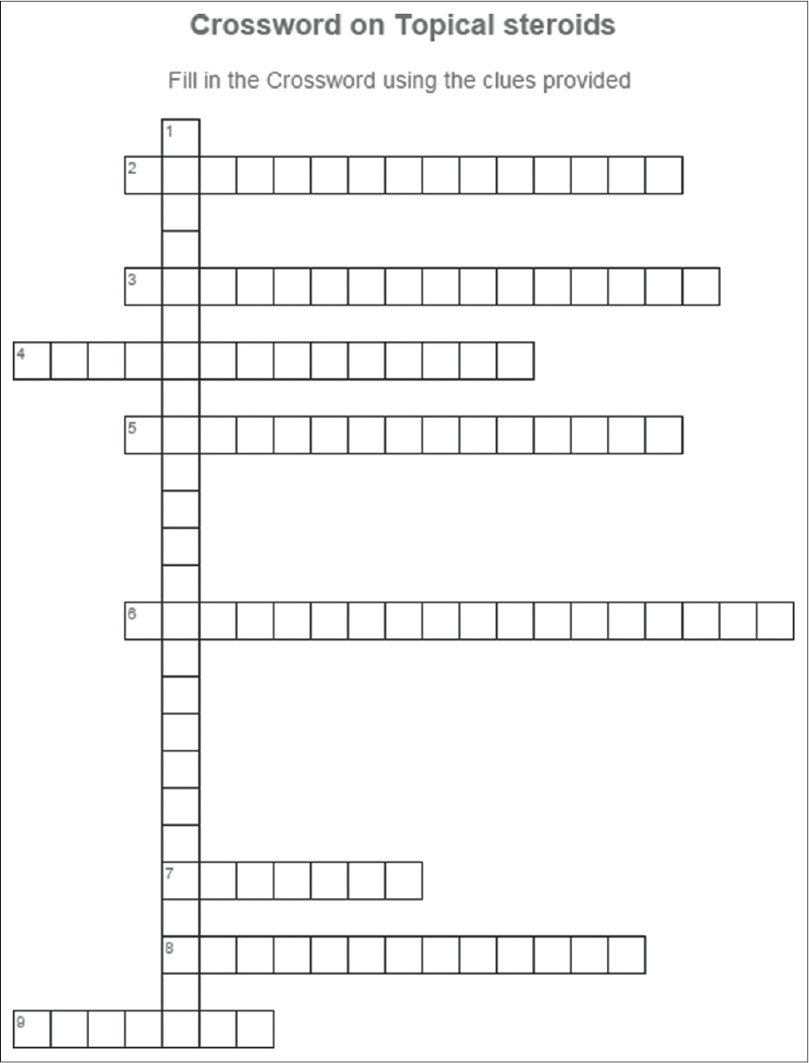 |
| Figure 2: Crossword on topical steroids |
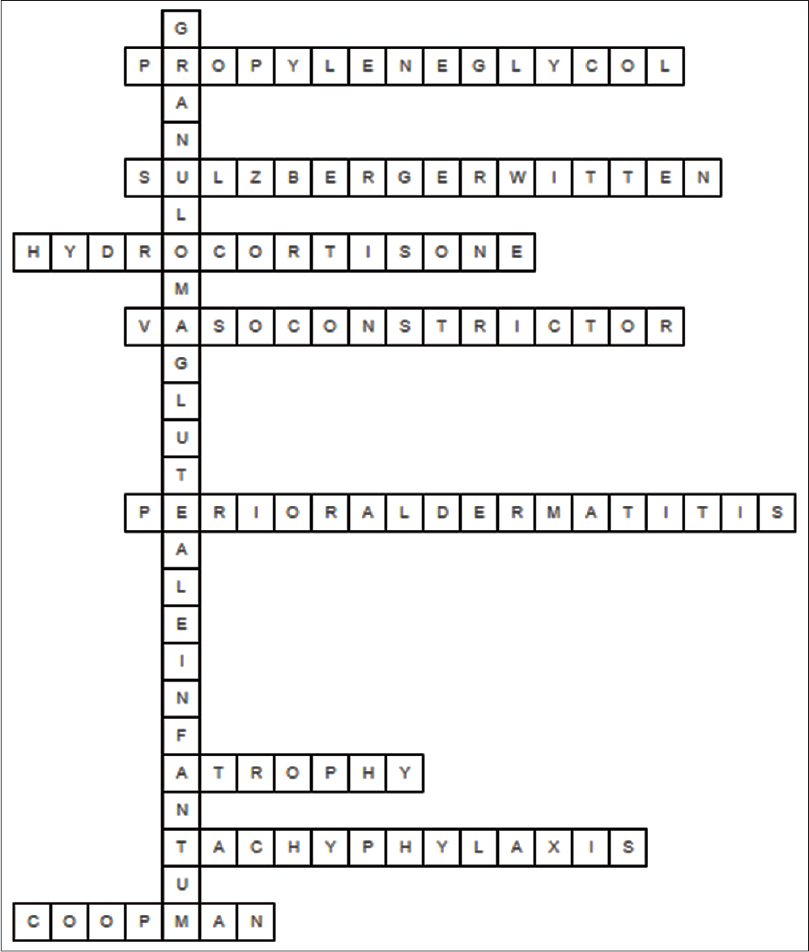 |
| Figure 3: Answers to the crossword on topical steroids |
When does one suspect contact hypersensitivity to topical steroids?
Contact hypersensitivity to topical steroids is suspected when there is persistence or worsening of skin disease on topical steroids. The risk increases when there is prolonged exposure to the drug. Non-fluorinated corticosteroids (e.g., hydrocortisone, hydrocortisone 17-butyrate and budesonide) result in higher prevalence of corticosteroid contact allergy in comparison to fluorinated compounds. This has to be distinguished from hypersensitivity to other constituents, for example, lanolin and preservatives such as parabens and antibiotics.
A classification scheme based on structural relation of the steroid molecule has been devised for cross-reactivity. This scheme with the representative agents hydrocortisone (group A), triamcinolone acetonide (group B), betamethasone (group C) and hydrocortisone butyrate (group D) is useful for clinical tests of contact allergy to corticosteroids.
DERMATOPHYTOMA
What is a dermatophytoma?
Dermatophytoma (dermatophyte fungal ball) is an uncommon clinical manifestation of onychomycosis caused by dermatophytes.
Dermatophytoma appears as linear, single, or multiple white or yellow bands on the nail plate and can be easily diagnosed from external appearances. It is characterized by abundant fungal filaments, large spores, or both, compacted and forming a fungal ball. Biofilm development is proposed for the pathogenesis of this infection. The fungal mass firmly attaches to the nail plate and produces an extracellular polysaccharide and this leads to decreased antifungal penetration making the condition resistant to standard antifungal therapy.
Definitive diagnosis is made microscopically by observation of this fungal conglomerate via direct potassium hydroxide or chlorazol black E examination of nail samples from patients with suspected onychomycosis. Oral antifungal treatment alone is not enough to treat this condition and either chemical or physical debridement is essential to eliminate dermatophytoma.
Livedoid Vasculopathy
What is livedoid vasculopathy?
Livedoid vasculopathy is a chronic, recurrent, occlusive vasculopathy of small dermal vessels characterized by recurrent, painful, slowly healing ulcerations on the lower extremities resulting in porcelain-white, stellate, atrophic scars surrounded by hyperpigmentation and telangiectasias, referred as "atrophie blanche of Milian." It predominantly affects young and middle-aged women. Focal, painful, purpuric macules and/or papules on the legs may be seen in early cases.
What are the other names for this condition?
Livedo vasculitis, segmental hyalinizing vasculopathy, livedo vasculitis with summer/winter ulceration and painful purpuric ulcers with reticular pattern on the lower extremities [PURPLE].
Why is the condition called livedoid vasculopathy and not "vasculitis?"
The histopathology shows segmental hyalinizing vascular involvement of thickened dermal blood vessels, endothelial proliferation and focal thrombosis without leukocytoclasia. No true vasculitis is evident. Direct immunofluorescence study reveals immunoglobulin and complement components in the superficial, mid-dermal and deep dermal vessels, but this is merely the result of sponge-like absorption of nonpathogenic immune components in the thickened vessels. Pathogenesis involves hyalinization and thrombosis rather than leukocytoclastic vasculitis.
What are the factors causing livedoid vasculopathy?
Formation of microthrombi in the superficial dermal vessels secondary to a defect in endothelial cell plasminogen activator, platelet dysfunction or enhanced fibrin formation are considered possible pathogenic mechanisms. Livedoid vasculopathy has been observed in patients with altered coagulation such as those with factor V Leiden mutation, protein C deficiency, antiphospholipid syndrome, increased plasma homocysteine levels, abnormalities in fibrinolysis, increase in platelet activation and sickle cell disease.
Outline the treatment options for livedoid vasculopathy
There is no single efficacious treatment for livedoid vasculopathy. Ethylestrenol, dapsone, corticosteroids, acetylsalicylic acid, clopidogrel, cilostazol, sildenafil, tadalafil, warfarin, low molecular weight heparin, nifedipine, nicotinic acid, pentoxifylline, S2 serotoninergic blockers, cyclosporine, hyperbaric oxygen, danazol, iloprost, intravenous immunoglobulin and psoralen plus ultraviolet-A have all been tried with some benefit.
CRYPTOCOCOCCOSIS
What are the causative organisms of cutaneous cryptococcosis?
Cryptococcosis is caused by two species of cryptococcus, Cryptococcus neoformans and Cryptococcus gattii. C. neoformans is associated with infections in the immunocompromised whereas C. gattii causes infections in the immunocompetent.
What is disseminated cryptococcosis?
Disseminated cryptococcosis is defined by a positive culture from at least two different sites or a positive blood culture. Risk factors for disseminated cryptococcosis include immunosuppression, malignancy, corticosteroid therapy, diabetes and connective tissue disease.
What is the mode of acquisition of cutaneous cryptococcosis?
Cutaneous cryptococcosis can be either primary or secondary.
Direct inoculation into the skin usually results in a solitary lesion on the uncovered parts with primary lymph node involvement.
Lung is the most common site of primary involvement and 90% of cases can be localized here. Hematogenous spread can cause multicentric lesions involving the covered body parts with deep dermal or subcutaneous lesions. Lesions may resemble molluscum contagiosum, or appear acneiform, nodular, herpetiform, cellulitic, or keloid-like.
What are the investigations for the diagnosis of cutaneous cryptococcosis?
Investigations in a case of cutaneous cryptococcosis include:
- Latex agglutination test in serum, cerebrospinal fluid and urine
- Enzyme-linked immunosorbent assay
- Direct preparation performed on a drop of serum/exudate placed on a slide to visualize large budding cells with capsules that stain positive with periodic acid-Schiff, mucicarmine and India ink preparation
- Tissue culture of skin biopsy specimen can be done
- Cutaneous disease is often presumed to be disseminated, hence an appropriate workup for systemic involvement is essential. This includes a thorough history and physical examination, chest radiography or computed tomography scanning to evaluate pulmonary involvement, lumbar puncture and imaging of the central nervous system and other studies as indicated
- On histologic sections, organisms can be seen as oval, thick-walled spherule surrounded by a polysaccharide capsule. Special staining with methylene blue, alcian blue or mucicarmine may be performed to demonstrate the capsule. There can be two patterns of involvement. The first is the gelatinous type which shows numerous budding yeasts in a foamy stroma with some or no inflammation. The second is the granulomatous type which shows fewer, smaller organisms and a granulomatous inflammatory infiltrate.
What is the treatment of cutaneous cryptococcosis?
In immunocompetent patients, disseminated, non-central nervous system cryptococcus infection can be treated with oral fluconazole for 3-6 months or with itraconazole for 6-12 months. Central nervous system involvement is treated with intravenous amphotericin B combined with flucytosine followed by oral fluconazole.
In immunosuppressed patients, the initial treatment is similar but lifelong maintenance treatment with fluconazole may be required. Primary cutaneous disease can be treated with oral fluconazole or itraconazole.
FINASTERIDE
What is the mechanism of action of finasteride and dutasteride?
Testosterone is converted into its active form dihydrotestosterone by the type II alpha reductase enzyme. Dihydrotestosterone is a more potent androgen and is one of the etiological factors in patterned hair loss. Finasteride is a synthetic 4-azasteroid compound and a potent and selective type II alpha reductase inhibitor. Dutasteride is a combined type 1 and 2 5α-reductase inhibitor. It produces a dose-dependent reduction in serum and scalp dihydrotestosterone levels to a greater degree than finasteride.
What is the dosage of finasteride used in patterned hair loss in men and women?
Finasteride has been tried in several doses ranging from 0.2 to 5 mg, but 1 mg/day is the optimal dose for male pattern hair loss. Oral finasteride in doses of 2.5 mg/day or more may be effective for the treatment of female pattern hair loss in postmenopausal women, in the absence of clinical or laboratory signs of hyperandrogenism.
The dose of dutasteride varies from 0.15 to 0.5 mg in different studies of patterned hair loss.
What are the sexual adverse effects of finasteride?
Sexual adverse effects with finasteride occur at the rate of 2.1-3.8% (comparable to placebo). Erectile dysfunction is the most common side effect followed by ejaculatory dysfunction and loss of libido. These effects commonly occur early in therapy and are reversible, they return to normal on stoppage of the drug or with continual use of the drug over a period of time. Very few studies have reported prolonged sexual adverse effects and alterations in sperm morphology.
In which subset of patients of patterned hair loss should finasteride be used with caution?
Finasteride should be used with caution or avoided in patients who have had a history of oligospermia or infertility, particularly if they are newly married and trying to raise a family. A patient who is anxious and expresses reservations about taking the drug may also be offered other treatments.
What is the effect of finasteride on prostate-specific antigen levels?
Finasteride can cause a reduction in serum prostate-specific antigen levels which could therefore result in an underestimation of prostatic cancer risk. A 50% reduction in prostate-specific antigen levels has been seen in older men. Though prostate-specific antigen levels remain valid while patients are taking finasteride, the value should be doubled to correct for the finasteride effect.
Erythema Gyratum Repens
Describe the clinical features of erythema gyratum repens
Erythema gyratum repens is a figurate erythema with a characteristic wood grain pattern and rapid peripheral spread. Polycyclic scaly erythematous plaques which produce concentric, figurate erythematous eruptions are characteristic and these migrate by 1 cm daily. The scales are usually present at the trailing edge and itching is usually present. Ichthyosis or bullae may be seen. Palmar hyperkeratosis may be present.
What is the pathogenesis of erythema gyratum repens?
Erythema gyratum repens has a strong association with internal malignancy in over 80% of cases. It is considered as an immunologically driven skin disease, however, its etiology is poorly understood. The three hypotheses regarding its pathogenesis include:
- Cross-reacting tumor antigens which causes an inflammatory skin disorder
- Transformation of normal skin proteins by tumor thus making them antigenic
- Deposition of immune complexes with tumor antigens at the basement membrane zone and induction of inflammation.
Enumerate the diseases which are associated with erythema gyratum repens?
Erythema gyratum repens is considered to be paraneoplastic and is commonly associated with lung cancer; other malignancies include cancers of esophagus, breast, bowel, uterus, cervix, kidney, pancreas and hematological neoplasia. Some non-parneoplastic cases may be idiopathic or have been reported in association with tuberculosis, cryptogenic pneumonia and CREST syndrome.
Enumerate the four classical annular erythemas
The four classical annular erythemas include:
- Erythema chronicum migrans
- Erythema marginatum rheumaticum
- Erythema gyratum repens
- Erythema annulare centrifugum.
GOUT
What are the four stages of gout?
Gout is a systemic disorder caused due to abnormal uric acid metabolism. Uric acid crystallizes and gets deposited in the joints resulting in recurrent arthritis. There are four stages of gout: asymptomatic hyperuricemia, gouty arthritis, inter-critical period and chronic gouty arthritis.
What are gouty tophi?
Chronic cutaneous gout is characterized by firm, erythematous nodules called tophi which are present intradermally or in the subcutis. They usually occur in avascular tissue on the ears, olecranon and prepatellar bursae, or on acral areas around the joints.
Atypical forms of tophaceous gout include bullous, fungating and ulcerative gout. Gouty panniculitis and miliarial gout are other variants. Miliarial gout is characterized by multiple tiny, painless, white-to-yellow colored papules on erythematous areas.
What are the microscopic findings seen in gouty tophi?
On histopathology, gouty tophi are seen as amorphous, pinkish, crystalline material in the dermis surrounded by granulomatous inflammation. These can also be seen under polarized light microscopy which shows negatively birefringent urate crystals with typical needle-like shapes.
Enumerate the differential diagnosis of gout
Differential diagnoses include pseudogout (calcium pyrophosphate deposition disease), a close mimicker of gout, multicentric reticulohistiocytosis, rheumatoid arthritis and psoriatic arthropathy.
Vulvovaginal Gingival Syndrome
What is vulvovaginal gingival syndrome?
Vulvovaginal gingival syndrome or Hewitt-Pelisse syndrome is a rare, severe variant of lichen planus.
It is characterized by erosions and desquamation of the vulval, vaginal and gingival mucosae and can result in scarring and the formation of strictures. Rarely, conjunctivae, lacrimal glands, auditory canal and esophagus can also be involved. Other findings include scarring alopecia and nail changes, cutaneous lesions may be associated in some cases. The probability of malignancy in these cases is 0.5-5%.
Enumerate various differential diagnoses of vulvovaginal gingival syndrome.
Differential diagnoses include pemphigus vulgaris, lichen sclerosus, leukoplakia, chronic candidiasis, Behcet′s disease and mucosal discoid lupus erythematosus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Fulltext Views
5,069
PDF downloads
2,110





