Translate this page into:
Vulvovaginal-gingival syndrome and esophageal involvement in lichen planus
2 Department of Pathology, Armed Forces Medical College and Command Hospital, Pune, Maharashtra, India
Correspondence Address:
Biju Vasudevan
Department of Dermatology, Command Hospital, Pune - 411 040, Maharashtra
India
| How to cite this article: Vasudevan B, Neema S, Verma R, Deb P, Kharayat V, Sethumadhavan T. Vulvovaginal-gingival syndrome and esophageal involvement in lichen planus. Indian J Dermatol Venereol Leprol 2016;82:209-211 |
Sir,
Lichen planus is a common dermatological disorder that usually manifests as violaceous, pruritic papules on the skin. It has many clinical variants which also involve the mucosa; oral mucosa is frequently affected, but vulvovaginal involvement is rather rare. The association of lichen planus of the vulva and vagina with desquamative gingivitis is described as the vulvovaginal-gingival syndrome. [1] We found only a few previous reports of this syndrome and even fewer with esophageal involvement. [2]
A 50-year-old woman presented with complaints of burning in the mouth and soreness of the genitalia for 4 years. She had multiple episodes of oral ulceration and increased redness of the gums for the past few months. Other mucosal symptoms including dysuria, dyspareunia, occasional ulceration and dysphagia were present for 1 year. She had only partially responded to the multiple topical and oral medications she had received.
General physical and systemic examination revealed no abnormalities. Dermatological examination revealed extensive erythema and desquamation over the gingiva [Figure - 1]a. The labial and vaginal mucosa had an erythematous, glazed appearance with excessive vaginal discharge [Figure - 1]b. Associated white lacy plaques were present on the buccal mucosa.
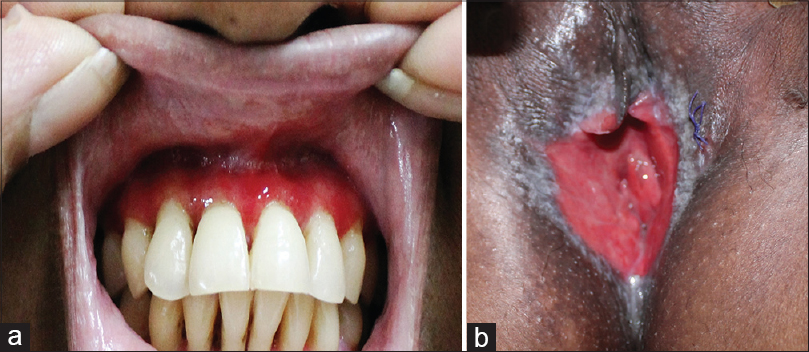 |
| Figure 1: Clinical findings: (a) Gingival erythema (b) Glazed erythema on the vulva |
On laboratory evaluation, she was detected to have microcytic hypochromic anemia (hemoglobin, 9.6 g%). Other hematological, biochemical and urinary parameters were normal. Antinuclear antibodies, ELISA for human immunodeficiency virus (HIV) infection, hepatitis B surface antigen and anti-hepatitis C antibodies were negative or non-reactive. Pelvic ultrasonogram was normal. Upper gastrointestinal endoscopy revealed multiple ulcers in the esophageal mucosa. A stricture was present, 26 cm from the proximal end beyond which the endoscope was not negotiable [Figure - 2].
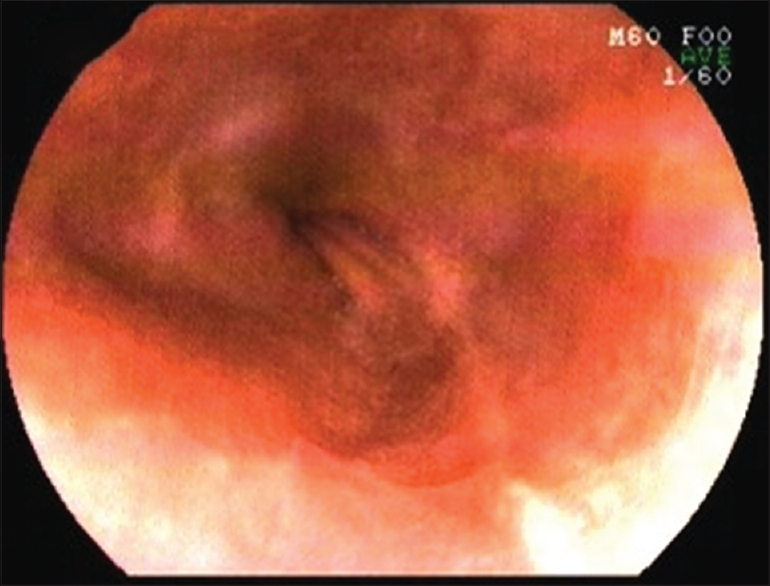 |
| Figure 2: Upper gastrointestinal endoscopy revealing ulceration of the esophageal mucosa |
Biopsies from the vulval and vaginal mucosa showed ulcerated epidermis, granulation tissue and an extensive inflammatory infiltrate [Figure - 3]a. There was saw-toothing of the rete ridges, basal cell vacuolation and an extensive band-like infiltrate in the upper dermis along the dermo-epidermal junction [Figure - 3]b. The infiltrate was predominantly lymphocytic in nature. Melanin incontinence was also noted. No atypical cells or other evidence of malignancy were present.
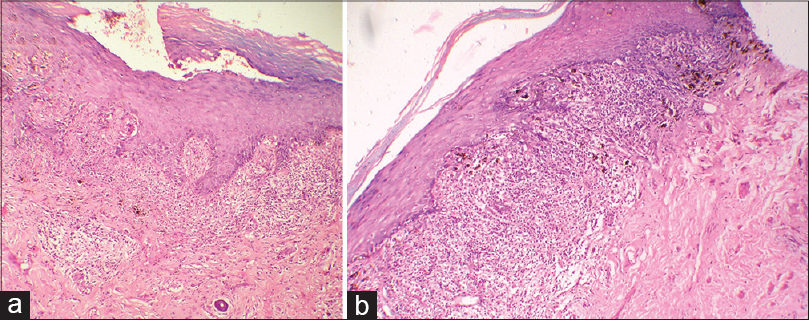 |
| Figure 3: Histopathology of vulval lesions (a) Extensive lymphocytic infiltrate along the dermo-epidermal junction and upper dermis with an absent basal layer (H and E, ×100) (b) Saw toothing of rete ridges and melanin incontinence (H and E, ×100) |
Biopsy from the buccal mucosa revealed similar features [Figure - 4]a. Biopsy from the esophagus revealed ulceration with extensive inflammatory infiltrate replacing the entire section [Figure - 4]b. Direct immunofluorescence in all sections revealed fibrinogen, immunoglobulin G, immunoglobulin M and C3 deposition at the dermo-epidermal junction. Thus, a diagnosis of vulvovaginal-gingival syndrome with esophageal involvement due to lichen planus was confirmed.
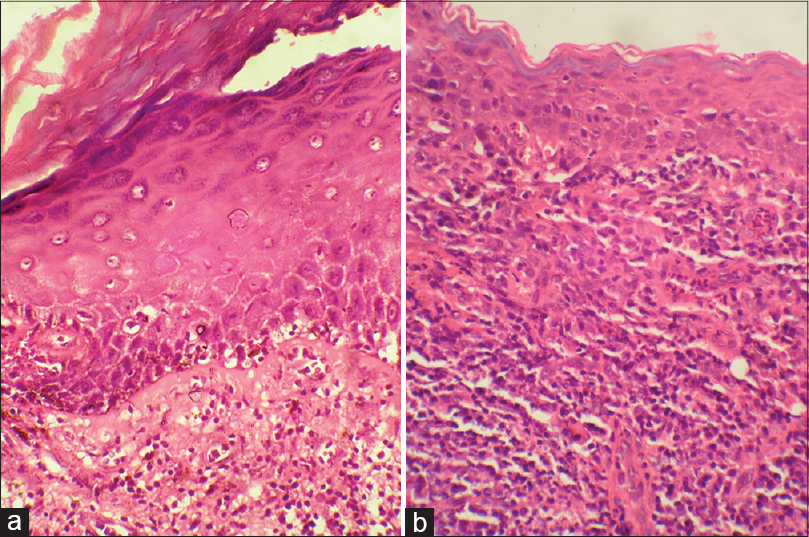 |
| Figure 4: (a) Histopathology of oral mucosa showing interface dermatitis with extensive infiltrate in upper dermis (H and E, ×100) (b) esophageal biopsy revealing absence of basal layer with extensive inflammatory infiltrate (H and E, ×400) |
She was initially managed with tapering doses of oral corticosteroids over 8 weeks besides topical treatment with 0.1% tacrolimus ointment on the oral and vaginal mucosae. Oral methotrexate was also added in a dose of 7.5 mg/week after 4 weeks, as a steroid-sparing agent for long-term therapy. She underwent serial esophageal balloon dilatation fortnightly on 6 ocassions to achieve a final controlled radial expansion of 12 mm. There was significant improvement in oral and vulval symptoms and she is presently able to swallow both solids and liquids comfortably.
Vulvovaginal-gingival syndrome or Hewitt-Pelisse syndrome, is a rare, severe variant of lichen planus which is characterized by erosions and desquamation of the vulval, vaginal and gingival mucosae and can result in scarring and the formation of strictures. [3] Rarely, conjunctivae, lacrimal glands, auditory canal and esophagus can also be involved. [4] Other findings include scarring alopecia and nail changes. [5] Strictures and stenosis have been reported in the vulval and vaginal mucosae. [6] Cutaneous lesions are also associated in a few cases but our patient had mucosal lesions alone. The probability of malignant conversion is 0.5-5%. [7],[8]
Unlike cutaneous lichen planus where the histopathological findings are characteristic, histopathological diagnosis is difficult in vulvovaginal-gingival syndrome as the epidermis is often ulcerated and this obscures the changes of lichen planus. For this reason, biopsy from the mucosa adjoining the ulcer is recommended. In case the biopsy findings are not specific, direct immunofluorescence examination to exclude vesiculobullous disorders such as pemphigus vulgaris and mucous membrane pemphigoid may be helpful. Other differential diagnoses include lichen sclerosus, leukoplakia, chronic candidiasis, Behcet′s disease and mucosal discoid lupus erythematosus. However, in our patient, all three mucosal sites revealed the classical features of lichen planus helping us to confirm the diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Setterfield JF, Neill S, Shirlaw PJ, Theron J, Vaughan R, Escudier M, et al. The vulvovaginal gingival syndrome: A severe subgroup of lichen planus with characteristic clinical features and a novel association with the class II HLA DQB1 FNx01 0201 allele. J Am Acad Dermatol 2006;55:98-113.
[Google Scholar]
|
| 2. |
Lewis FM. Vulval lichen planus. Br J Dermatol 1998;138:569-75.
[Google Scholar]
|
| 3. |
Pelisse M, Leibowitch M, Sedel D, Hewitt J. A new vulvovaginogingival syndrome. Plurimucous erosive lichen planus. Ann Dermatol Venereol 1982;109:797-8.
[Google Scholar]
|
| 4. |
Menges M, Hohloch K, Pueschel W, Stallmach A. Lichen planus with oesophageal involvement. A case report and review of the literature. Digestion 2002;65:184-9.
[Google Scholar]
|
| 5. |
Eisen D. The vulvovaginal-gingival syndrome of lichen planus. The clinical characteristics of 22 patients. Arch Dermatol 1994;130:1379-82.
[Google Scholar]
|
| 6. |
Mann MS, Kaufman RH. Erosive lichen planus of the vulva. Clin Obstet Gynecol 1991;34:605-13.
[Google Scholar]
|
| 7. |
Lewis FM, Harrington CI. Squamous cell carcinoma arising in vulval lichen planus. Br J Dermatol 1994;131:703-5.
[Google Scholar]
|
| 8. |
Franck JM, Young AW Jr. Squamous cell carcinoma in situ arising within lichen planus of the vulva. Dermatol Surg 1995;21:890-4.
[Google Scholar]
|
Fulltext Views
9,125
PDF downloads
2,415





