Translate this page into:
Wells syndrome in a patient receiving adalimumab biosimilar: A case report and review of literature
2 Department of Pathology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Correspondence Address:
Dipankar De
Department of Dermatology Venereology and Leprology, PGIMER, Chandigarh - 160 012
India
| How to cite this article: Dabas G, De D, Handa S, Chatterjee D, Radotra BD. Wells syndrome in a patient receiving adalimumab biosimilar: A case report and review of literature. Indian J Dermatol Venereol Leprol 2018;84:594-599 |
Sir,
Wells syndrome was first described by George Wells in 1971 as a recurrent granulomatous dermatitis with eosinophilia.[1] It presents with erythematous, indurated, tender or mildly pruritic plaques which mimic cellulitis. Demonstration of dermal edema, marked eosinophil infiltration and presence of free eosinophilic granules coating collagen bundles (“flame figures”) in histopathology is diagnostic.[1],[2]
A 36-year-old man with a 12-year history of hidradenitis suppurativa (Hurley's grade III) was started on adalimumab biosimilar (Exemptia; Zydus Cadila) monotherapy because of inadequate treatment response to multiple courses of antibiotics and retinoids (acitretin 35 mg/day for 1 year and 3 months). He presented to the outpatient department with three erythematous to plum colored indurated plaques of recent onset on the third and fifth digits of the right hand and sole of the right foot, 1 month after starting adalimumab biosimilar (6 hours after third dose) [Figure - 1],[Figure - 2],[Figure - 3]. He also complained of minimal pain that exacerbated with the movement of the fingers and on walking. There was no history of any other drug intake, fever, trauma, recent travel, insect bite or intravenous drug use. Sweet's syndrome, Wells syndrome and fixed drug eruption were considered as differential diagnoses.
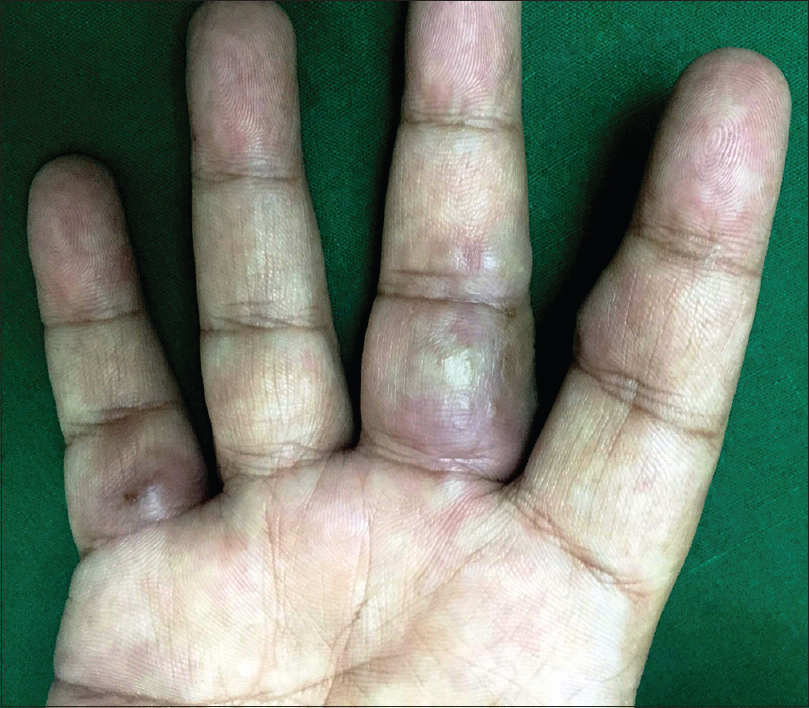 |
| Figure 1: Erythematous to plum-colored indurated plaques on the volar aspect of the third and fifth digits of the right hand |
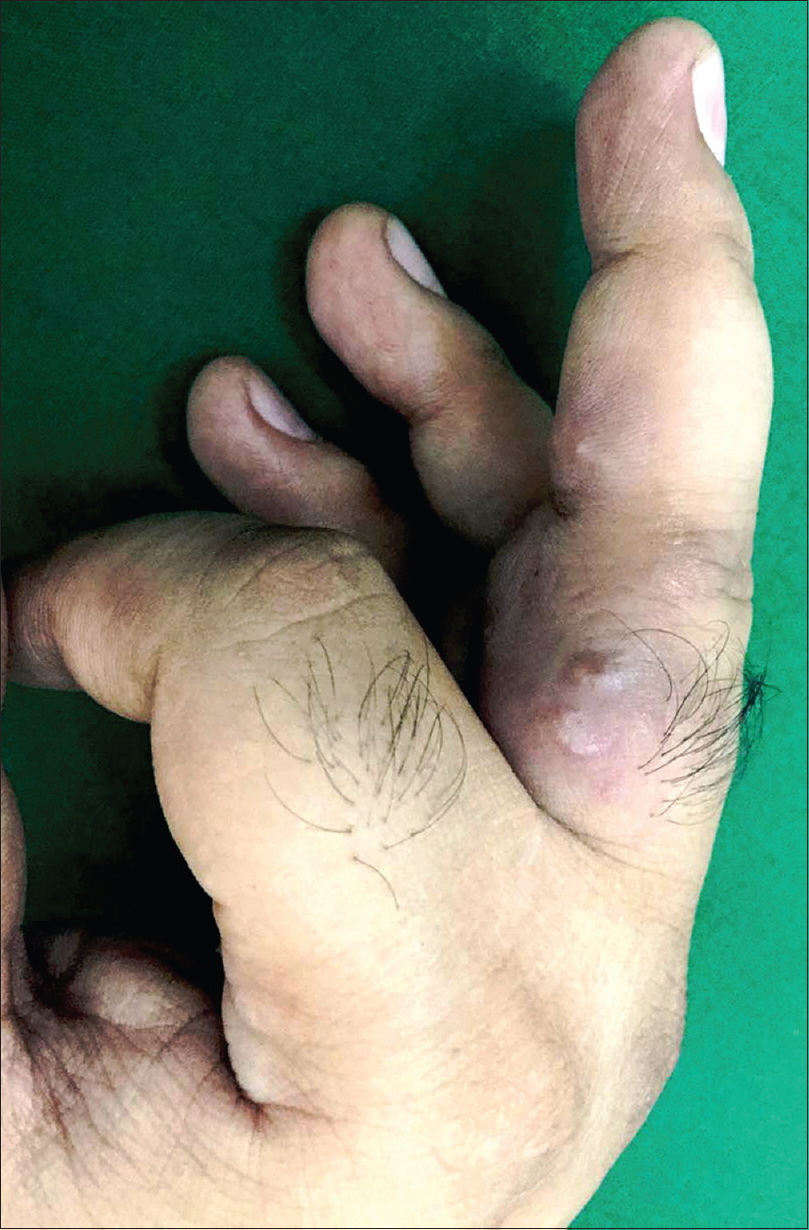 |
| Figure 2: Close-up view showing erythematous to plum-colored indurated plaque with overlying two small vesicles on the proximal and middle phalanges of the third digit of the right hand |
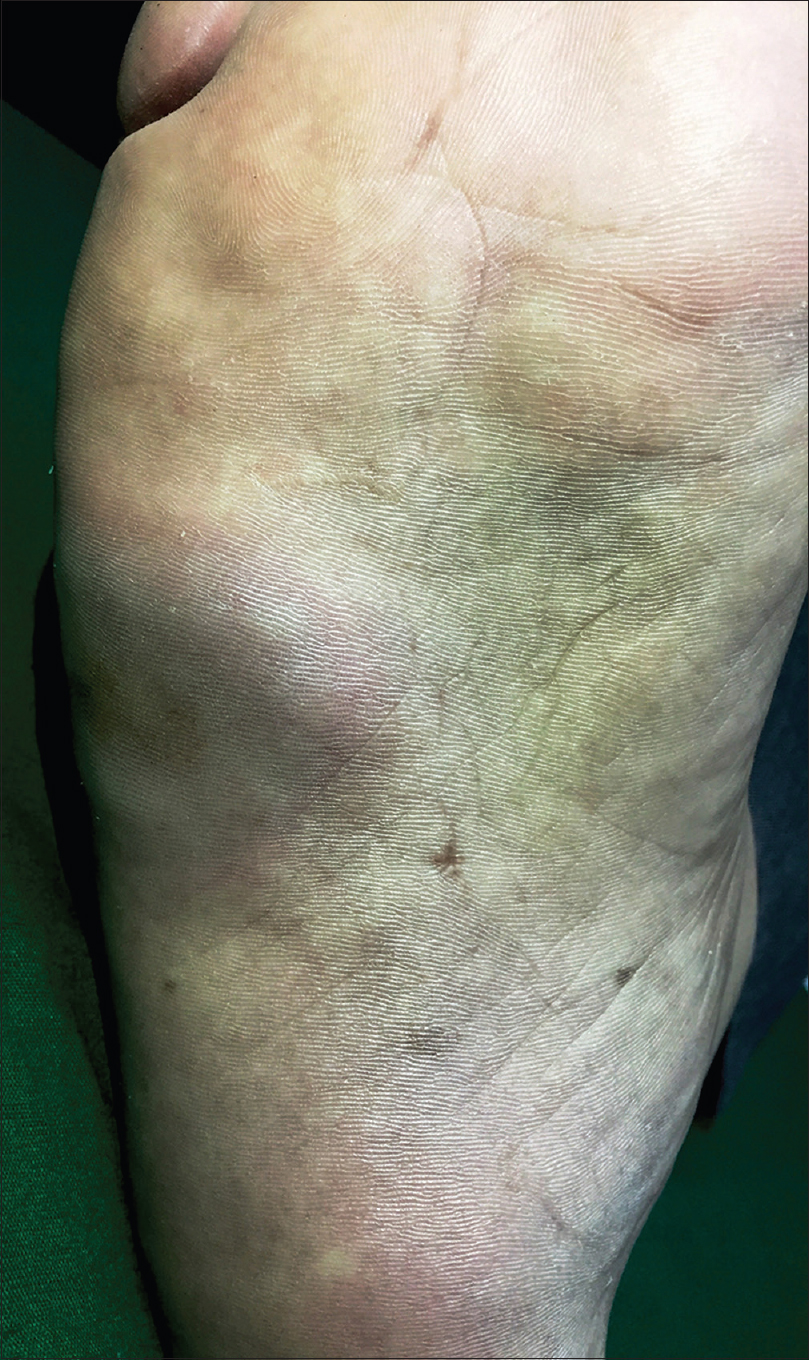 |
| Figure 3: Erythematous to plum-colored indurated plaque on the sole of the right foot |
A complete blood count revealed increased white blood cell count (13.37 × 103/dL [normal 4.00 × 103/dL to 11.00 × 103/dL]) and peripheral eosinophilia (18.6%) with an absolute eosinophil count of 2.48 × 103/dL (normal 0.10 × 103/dL to 0.50 × 103/dL). C-reactive protein levels (6.9 mg/L [normal 0 mg/L to 5.0 mg/L]) and ESR (70 mm/h) were found to be elevated, suggesting an inflammatory process. Total serum IgE 65 U/ml (2–100 U/ml), renal and liver function were normal. No ova or parasites were observed in stool microscopy. The clinical diagnosis of Wells syndrome was confirmed by the typical histopathological finding of marked eosinophil infiltration into the dermis and subcutaneous tissue with flame figures [Figure - 4] and [Figure - 5]. A short course of oral steroids, i.e. prednisolone 40 mg for 2 weeks, gradually tapered over the next 8 weeks led to disease resolution [Figure - 6]. Drug rechallenge test elicited similar results within 5 hours of giving the fourth dose. Adalimumab biosimilar was stopped and there has been no recurrence of the lesions thus far.
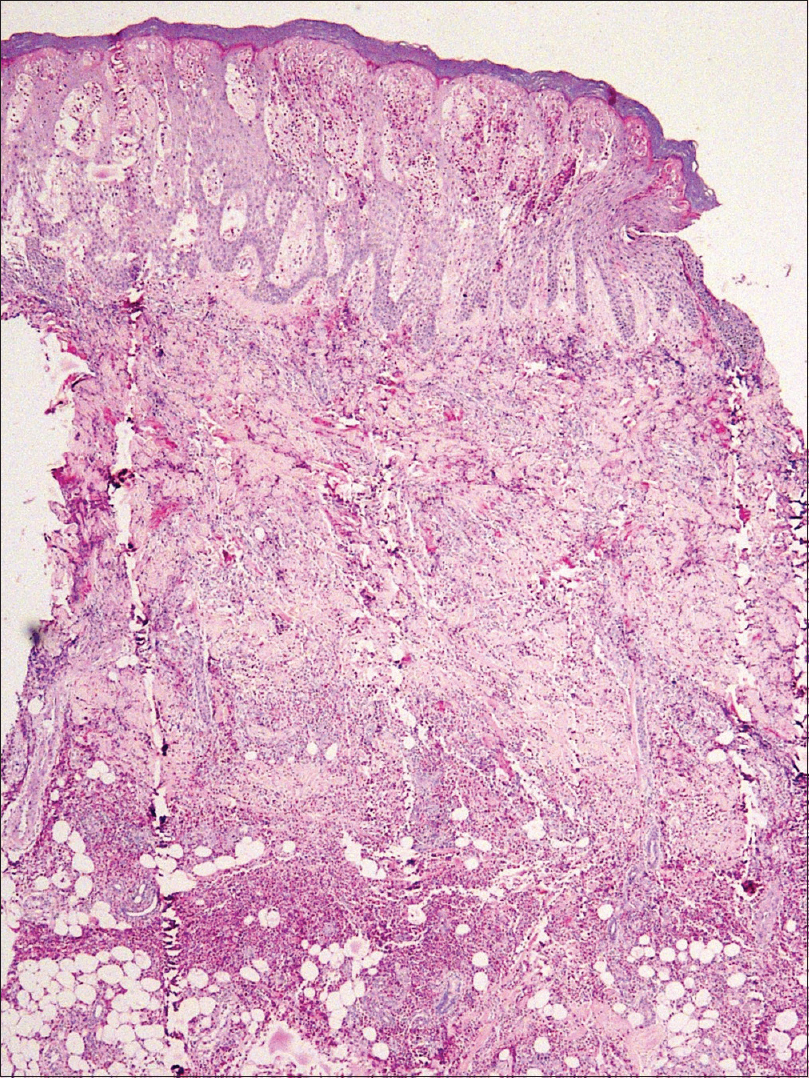 |
| Figure 4: Skin biopsy from the palm showing dense eosinophil infiltration extending from the epidermis till subcutaneous fat, with marked epidermal spongiosis [hematoxylin and eosin (H and E), ×40] |
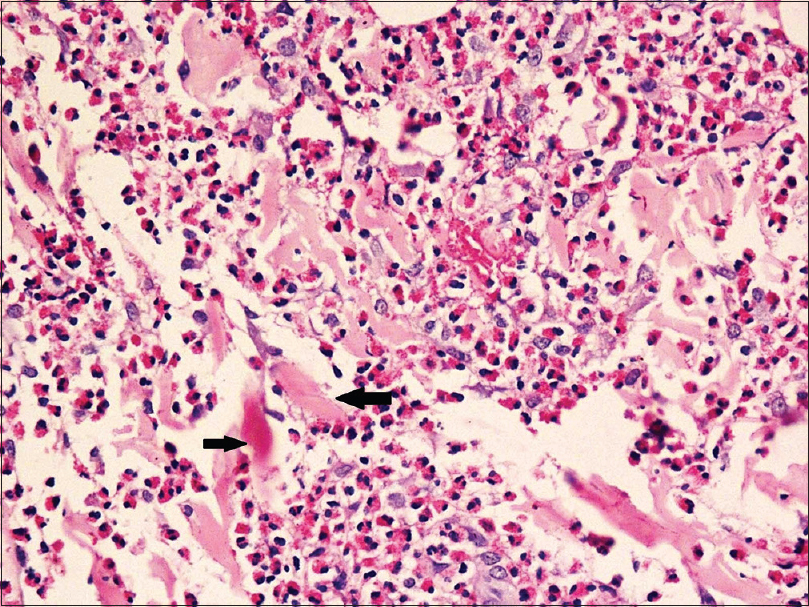 |
| Figure 5: Marked dermal edema and eosinophil infiltration with arrows showing flame figures (H and E, ×400) |
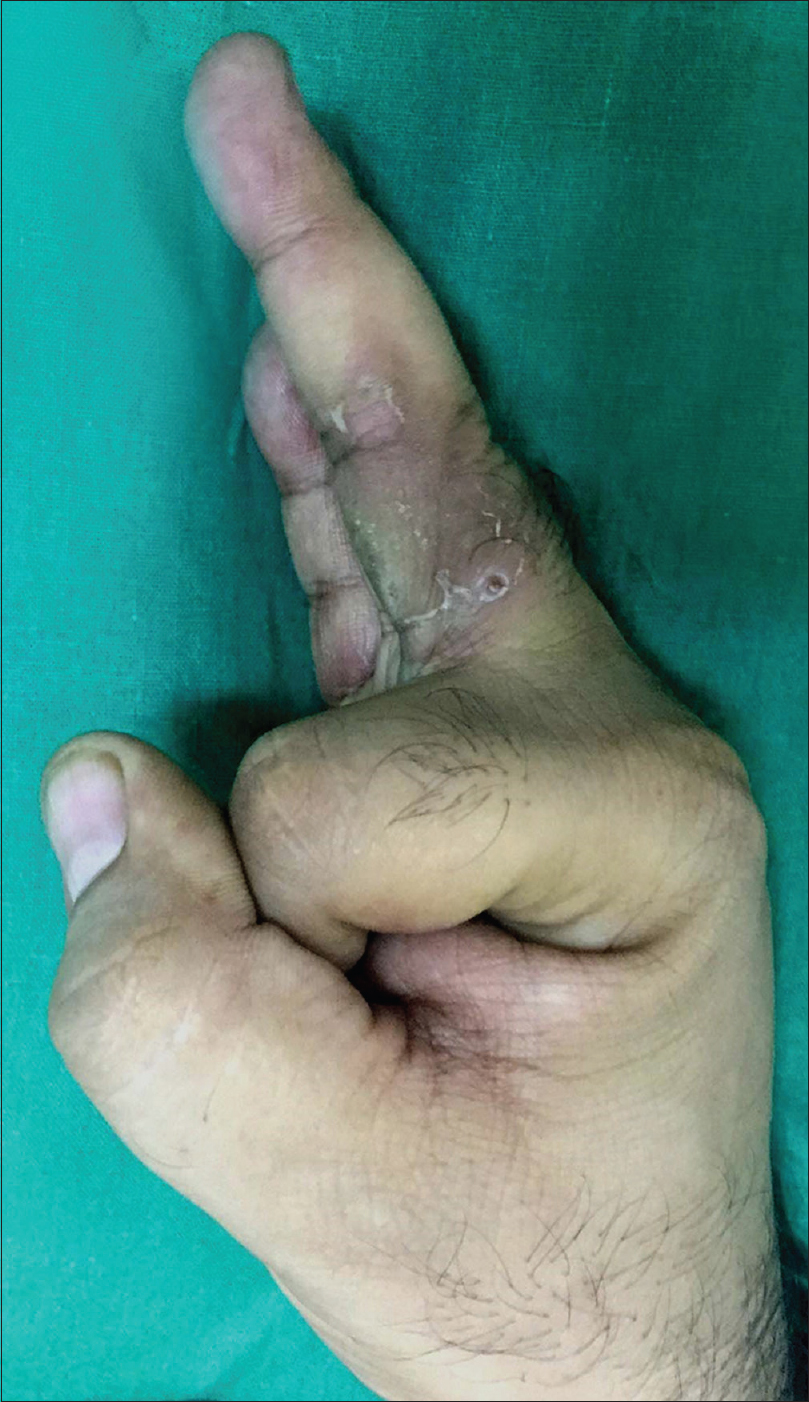 |
| Figure 6: Improvement in edema with desquamation over the lesions 6 weeks after starting prednisolone |
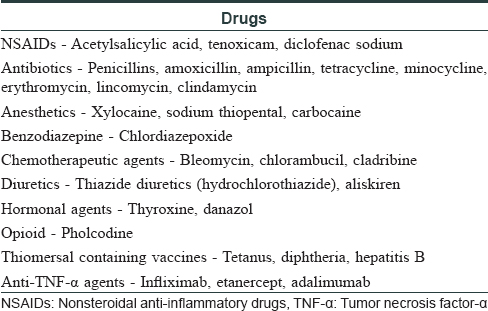
Wells syndrome presents with sudden onset, single or multiple, erythematous, indurated or urticarial plaques which resolve without scarring over 2–8 weeks. Clinical variants of Wells syndrome include plaque type, annular granuloma-like, urticaria-like, papulo-vesicular, bullous, papulo-nodular and fixed drug eruption-like lesions.[2] In our case, plaque-type lesions were seen. The pathogenesis is not very clear and it is believed to be a hypersensitivity reaction to a variety of exogenous and endogenous stimuli. Excessive production of interleukin-5 resulting in eosinophil accumulation and a local Th2 immune response has been documented. Wells syndrome is associated with infections (viral/parasitic/arthropod bite), malignancy, atopy, Churg–Strauss syndrome and drugs. [Table - 1] lists the iatrogenic causes of Wells syndrome.[2] Adalimumab-induced Sweet's syndrome, erythema multiforme and fixed drug eruption have been reported previously but the characteristic histopathology helped us in clinching the diagnosis.
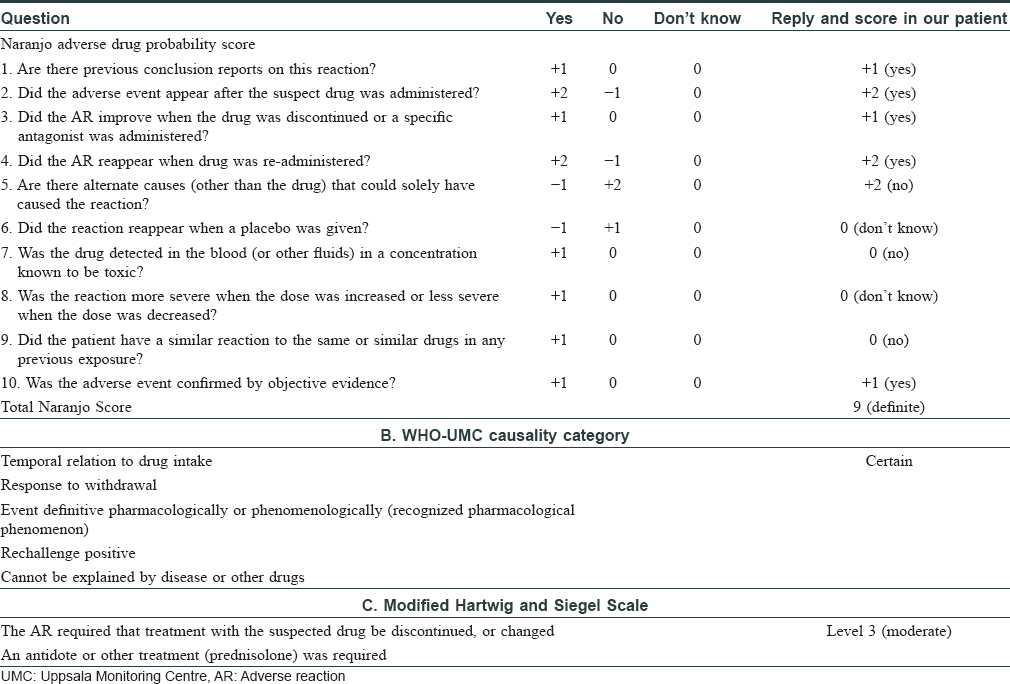
Some cases of eosinophilia associated with tumor necrosis factor-α inhibitors have been reported before.[3] Wells syndrome-like lesions have been described at the injection site of etanercept[4] and adalimumab[5] as local reaction to the drug, but not away from the injection site as observed in our patient. The Naranjo adverse drug probability score was 9 in our patient, suggestive of a definite adverse drug reaction [Table - 2]. A Medline and PubMed search was performed and we recorded the features in cases of Wells syndrome or cases with blood or tissue eosinophilia after anti-tumor necrosis factor-α therapy [Table - 3].[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15]
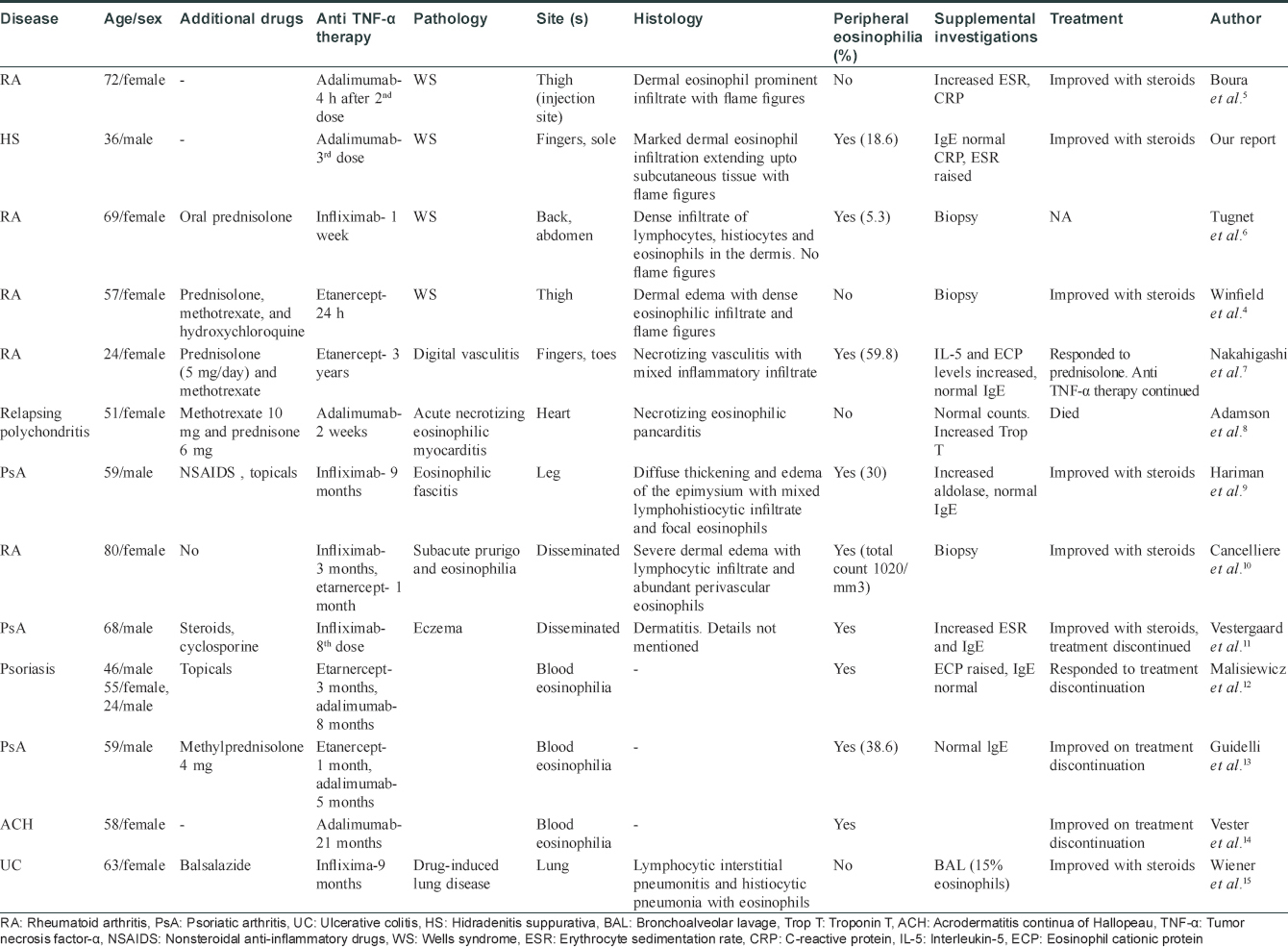
The mechanism of tumor necrosis factor-α inhibition leading to eosinophilia remains still elusive. Anti-tumor necrosis factor-α agents can induce an immune deviation from the Th1 to the Th2 phenotype in some patients, resulting in eosinophilia. Quaglino et al. reported that psoriasis patients responding to etanercept showed a significant reversal of the Th1/Th17 activation and a concomitant upregulation of Th2 and regulatory T cells.[16] Formation of IgE-class-switched antibodies can lead to IgE-mediated drug hypersensitivity and subsequent eosinophilia.[17] More sustained and effective tumor necrosis factor -α inhibition was associated with a higher risk of eosinophilia. Hence, adalimumab has more risk of causing eosinophilia than etanercept. Recently, Chiriac et al. reported a case series of 28 patients who developed eosinophilia during the first 6 months with adalimumab therapy.[18]
To conclude, the introduction of biologics has significantly improved treatment efficacy in several chronic inflammatory diseases. Recent reports suggest that tumor necrosis factor-α inhibitors can cause both blood and tissue eosinophilia. In most cases, it is benign and does not warrant drug withdrawal. However, reports of acute necrotizing eosinophilic myocarditis[8] and lung damage[15] due to tissue eosinophilia caused by tumor necrosis factor inhibitors also exist. It is important for the treating dermatologists to recognize this complication early and treat accordingly. With new biologics entering the market every year, it is vital to identify and report potential drug-related side effects.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that the names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971;57:46-56.
[Google Scholar]
|
| 2. |
Consigny S, Courville P, Young P, Richard C, Gauthier V, Maillard V, et al. Histological and clinical forms of the eosinophilic cellulitis. Ann Dermatol Venereol 2001;128:213-6.
[Google Scholar]
|
| 3. |
Beuthien W, Mellinghoff HU, von Kempis J. Skin reaction to adalimumab. Arthritis Rheum 2004;50:1690-2.
[Google Scholar]
|
| 4. |
Winfield H, Lain E, Horn T, Hoskyn J. Eosinophilic cellulitislike reaction to subcutaneous etanercept injection. Arch Dermatol 2006;142:218-20.
[Google Scholar]
|
| 5. |
Boura P, Sarantopoulos A, Lefaki I, Skendros P, Papadopoulos P. Eosinophilic cellulitis (Wells' syndrome) as a cutaneous reaction to the administration of adalimumab. Ann Rheum Dis 2006;65:839-40.
[Google Scholar]
|
| 6. |
Tugnet N, Youssef A, Whallett AJ. Wells' syndrome (eosinophilic cellulitis) secondary to infliximab. Rheumatology (Oxford) 2012;51:195-6.
[Google Scholar]
|
| 7. |
Nakahigashi K, Egawa G, Miyachi Y, Kabashima K. Digital vasculitis with eosinophilia possibly associated with etanercept therapy. Acta Derm Venereol 2014;94:239-40.
[Google Scholar]
|
| 8. |
Adamson R, Yazici Y, Katz ES, Greisman SG, Steiger D. Fatal acute necrotizing eosinophilic myocarditis temporally related to use of adalimumab in a patient with relapsing polychondritis. J Clin Rheumatol 2013;19:386-9.
[Google Scholar]
|
| 9. |
Hariman R, Patel P, Strouse J, Collins MP, Rosenthal A. Development of eosinophilic fasciitis during infliximab therapy for psoriatic arthritis. Case Rep Rheumatol 2016;2016:7906013.
[Google Scholar]
|
| 10. |
Cancelliere N, Barranco P, Vidaurrázaga C, Benito DM, Quirce S. Subacute prurigo and eosinophilia in a patient with rheumatoid arthritis receiving infliximab and etanercept. J Investig Allergol Clin Immunol 2011;21:248-9.
[Google Scholar]
|
| 11. |
Vestergaard C, Deleuran M, Kragballe K. Two cases of atopic dermatitis-like conditions induced in psoriasis patients treated with infliximab. J Eur Acad Dermatol Venereol 2007;21:1272-4.
[Google Scholar]
|
| 12. |
Malisiewicz B, Murer C, Pachlopnik Schmid J, French LE, Schmid-Grendelmeier P, Navarini AA, et al. Eosinophilia during psoriasis treatment with TNF antagonists. Dermatology 2011;223:311-5.
[Google Scholar]
|
| 13. |
Guidelli GM, Tenti S, Fioravanti A. Severe eosinophilia during anti-tumor necrosis factor-alpha therapy for psoriatic arthritis. Indian J Dermatol Venereol Leprol 2014;80:187-9.
[Google Scholar]
|
| 14. |
Vester K, Rüger RD, Harth W, Simon JC. Transient blood eosinophilia during treatment with adalimumab. J Eur Acad Dermatol Venereol 2012;26:924-5.
[Google Scholar]
|
| 15. |
Wiener CM, Muse VV, Mark EJ. Case records of the Massachusetts General Hospital. Case 33-2008. A 63-year-old woman with dyspnea on exertion. N Engl J Med 2008;359:1823-32.
[Google Scholar]
|
| 16. |
Quaglino P, Bergallo M, Ponti R, Barberio E, Cicchelli S, Buffa E, et al. Th1, th2, th17 and regulatory T cell pattern in psoriatic patients: Modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response. Dermatology 2011;223:57-67.
[Google Scholar]
|
| 17. |
Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, Pennino D, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med 2011;365:231-8.
[Google Scholar]
|
| 18. |
Chiriac A, Brzezinski P, Stolnicu S, Podoleanu C, Moldovan C, Molnar C, et al. Eosinophilia � A rare possible adverse reaction during anti-tumor necrosis factor-alpha therapy for psoriasis. J Dermatolog Treat 2016;27:110-3.
[Google Scholar]
|
Fulltext Views
4,815
PDF downloads
1,752





