Translate this page into:
Western blot profile in HIV infection
Correspondence Address:
V Lakshmi
Department of Microbiology, Nizam's Institute of Medical Sciences, Punjagutta, Hyderabad - 500 082, Andhra Pradesh
India
| How to cite this article: Sudha T, Lakshmi V, Teja V D. Western blot profile in HIV infection. Indian J Dermatol Venereol Leprol 2006;72:357-360 |
Abstract
Background: Although the overall sensitivity and specificity of the western blot (WB) test for detection of antibodies to various viral proteins is high, there has been a substantial difference in the timing of the appearance of antibody bands and their intensities during different stages of HIV infection. Aims: Mapping different band patterns of Western blot results and correlating them with stages of HIV infection. Methods: We performed a retrospective study with 1,467 HIV-1 infected cases confirmed by WB test between January 2002 to July 2005, with the objective of mapping different band patterns of western blot results and determining whether the presence or absence of certain bands was associated with any specific stage of HIV infection. For the interpretation of the WB results in this study, the guidelines recommended by NACO, India were followed. Results: Reactivity with all the bands was the most commonly observed WB pattern, occurring in 92.91% (1363/1467) of cases, whereas the other 7.09% showed uncommon band patterns. Of all individual bands, p31 band was the most frequently missing one, absent in 7.09% cases. On classifying the WB reactive cases by the WHO clinical staging system, 38.45% (564/1467) were in Stage 1, 47.99% (704/1467) in stages 2 and 3 and 13.56% in stage 4. Correlation of CD4 cell counts with the various uncommon band patterns showed that only 5.56% (4/72) had counts in the 200-500 cells/�l range, whereas 45.83% and 48.61% had counts of <200 and >500 cells/�l respectively. Conclusion: Interpretation of the WB band pattern in combination with clinical features may be occasionally useful in predicting the stage of HIV infection.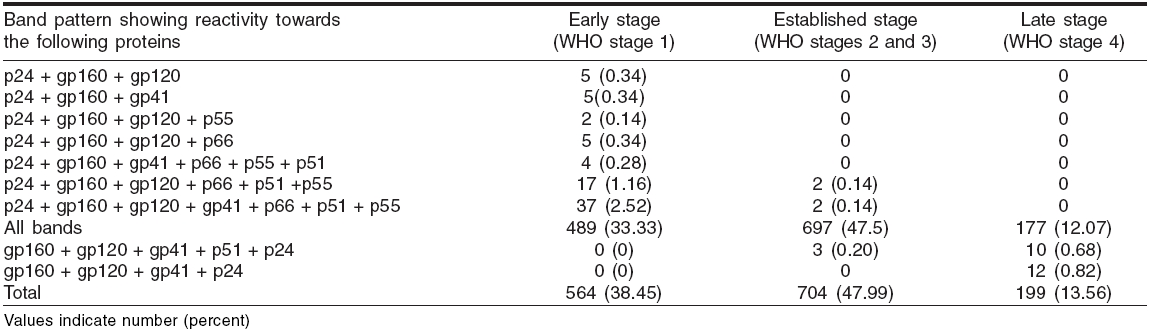

 |
| CD4 cell counts as observed in the uncommon western blot band patterns |
 |
| CD4 cell counts as observed in the uncommon western blot band patterns |
 |
| Cases with the absence of reactivity towards various proteins on western blot test |
 |
| Cases with the absence of reactivity towards various proteins on western blot test |
Introduction
Current laboratory diagnosis of human immunodeficiency virus (HIV) infection is mainly based on the detection of anti-HIV antibodies.[1] HIV tests have become progressively more sensitive and specific with the use of recombinant and/or synthetic peptide antigens as the basis of the tests.[2] Western blotting is one of the reference confirmatory tests for the diagnosis of HIV infection or after inconclusive EIA results.[3],[4],[5]
Although the overall sensitivity and specificity of the WB for detection of antibodies to the various viral proteins is high, there have been substantial differences in the timing of the appearance of antibody bands and their intensities during different stages of HIV infection. However, there have been no large-scale studies that can provide any significant findings from the band patterns of WB tests. We performed a retrospective study with the objective of mapping different band patterns of WB results of HIV-1 reactive individuals.
Methods
During a period of January 2002 to July 2005, a total of 1,669 WB tests were performed on sera with two reactive HIV ELISA tests in the Department of Microbiology, Nizam′s Institute of Medical Sciences, a tertiary care hospital and university. All these samples were of patients attending different departments of our institute. Western blot tests were performed using two commercially available licensed kits - HIV Blot 2.2 (Genelabs, Singapore) and HIV W. Blot (J. Mitra and Co. Ltd, New Delhi).
For the interpretation of the WB results in this study, the guidelines recommended by NACO, India[6] were followed. A WB was considered positive if at least two of the three bands representing the envelope bands (gp160, gp120 and gp41 of HIV-1 and gp36 of HIV-2) and gag gene p24 were present. The absence of all the bands except the control one was considered as a negative test. Presence of any nonspecific band or isolated p17 or p55 was considered negative.[7],[8] Western blot results that could not be classified as negative or positive were categorized as indeterminate.
Out of the 1,669 serum samples tested for WB, 87 were nonreactive, 14 were indeterminate and 101 were either reactive for HIV-1 and HIV-2 or HIV-2 alone, leaving 1,467 confirmed cases of HIV-1 alone for the study. The band patterns of these 1,467 HIV-1 positive samples were further mapped with respect to different HIV infection stages and the corresponding CD4 counts. CD4 cell counts were performed on FACS count system (Beckton and Dickinson).
All the demographic, clinical and diagnostic data of WB positive cases was collected from a review of their medical records, which are stored in our Institute′s Medical Record Division.
Results
In order to quantify the contribution of this reactivity level to the prediction of the HIV status based on the clinical stage, we classified the band reactivity pattern into three categories-early sero-conversion stage, established infection stage and advanced HIV-1 infection [Table - 1].
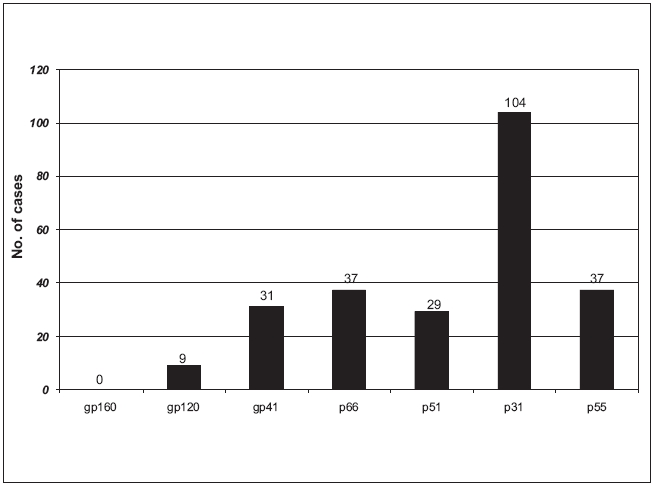
Reactivity with all the bands was the most commonly observed WB pattern, occurring in 92.91% (1363/1467) of samples; the other 7.09% showed uncommon band patterns [Table - 1]. The most prevalent one was gp160 as it was observed in all the reactive cases. All the three envelope proteins were observed in 96.86% samples, whereas all the three polymerase and core proteins were observed in 92.91% and 72.19% samples respectively. Of all individual bands, p31 band was the most frequent missing band, being absent in 7.09% samples, followed by other bands like p55, p66, p51, p41, etc. [Figure - 1].
On classifying these WB reactive cases by the WHO clinical staging system, 38.45% (564/1467) were in Stage 1, 47.99% (704/1467) in stages 2 and 3 and 13.56% in stage 4 [Table - 1].
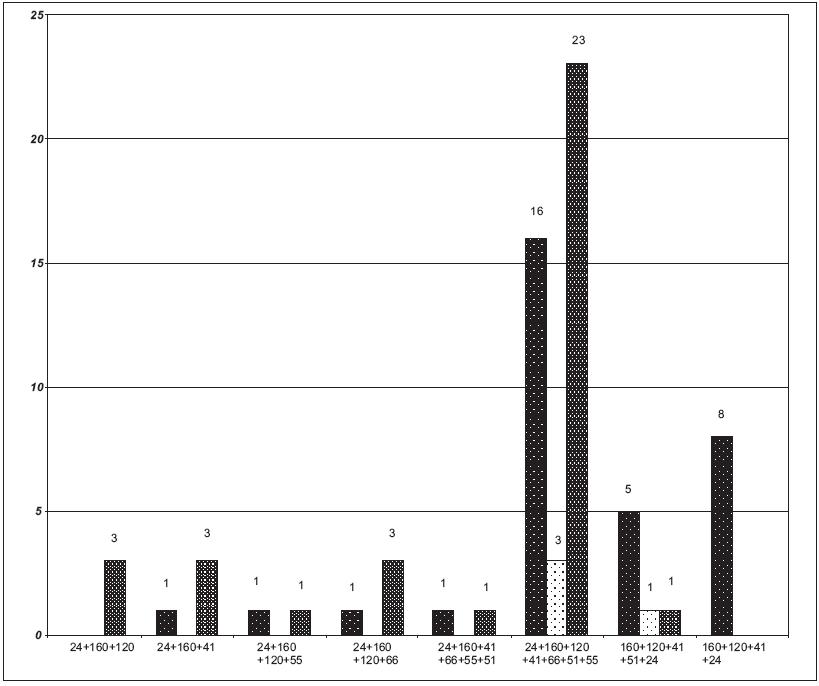
Out of 104 samples showing uncommon WB profiles, 72 samples were also screened for CD4 cell counts. The correlation of CD4 cell counts with these band patterns showed that only 5.56% (4/72) had cell counts in the 200-500 cells/µl range, whereas 45.83% and 48.61% had cell counts of < 200 and> 500 cells/µl respectively [Figure - 2].
Discussion
A positive WB test after two reactive ELISA tests continues to be the gold standard for diagnosis of HIV infection. We observed 92.91% cases with reactivity towards all the proteins in all of the four WHO stages. While this finding did not help identify the stage of HIV infection, the other uncommon (7.09%) WB profiles such as the absence of certain bands were more useful in this regard. It was observed that a WB profile showing reactivity towards the HIV-1 viral gag protein p24 with env glycoproteins gp160 and/or gp120 and 41, together with any of the pol proteins, but with the absence of p31, can be used as a predictor of sero-conversion [Table - 1]. Similarly, the presence of all envelope glycoproteins gp160, gp120 and gp 41, with p24 and/or p51, but with absence of all other bands, can predict the late stage of HIV infection. This band pattern was similar to that seen in the early stage, the major difference being that in the late stage all env glycoproteins and p24 were present and all other protein bands were absent, while in the early stage p24 was present with only one or two env glycoproteins in combination with or without pol genes.
The p31 reactive band was the most frequently undetectable protein in our study unlike other studies where gp41 and p55 were the most frequently missing bands.[9],[10] In contrast, antibodies to the envelope glycoprotein gp160 and its cleavage products gp120 and gp41 were detected in specimens from virtually all HIV-infected persons regardless of the clinical stage, as in other studies.[11] One hundred and four samples (7.09%) with a missing p31 reactive band were exclusively observed to be either in the early stage or in the late stage of infection, suggesting that the lack of antibody reaction to p31 with or without other protein bands is a predictor for the early or the late stage of infection.
On correlation of CD4 cell counts with the uncommon WB band patterns, it was observed that out of the 33 samples (45.83%) where the cell counts were < 200 cells/µl, 20 samples were in the early stage of infection. However, this could have resulted from their being in the acute sero-conversion stage which may have depressed their CD4 cell counts [Figure - 2]. Similarly, except for one sample, all the samples of uncommon WB band patterns with CD4 counts of > 500 cells/µl were in the early stage of infection.
Thus, in situations where patients cannot afford the costlier viral load test and CD4 cell counts, the WB pattern together with clinical symptoms may aid, at least in individuals with uncommon WB profiles, in predicting the stage of HIV infection.
One limitation of the study was that for a period of five months HIV W. Blot (J. Mitra and Co. Ltd., New Delhi) tests were used that did not show a good reactivity towards the p17 protein. This problem was later rectified. Since it was observed from the analysis of all other bands that the presence or absence of the p17 band did not aid in predicting the stage of HIV infection, the data of p17 band was not included in the analysis.
The p31 band was seen to be specific for HIV infection. It was observed to develop in the established stage of infection and disappear in the very late stages of infection along with the other gag and pol genes. As the identification of the stage of early HIV infection can be useful for clinical care and prevention efforts, interpretation of the WB band pattern in combination with clinical features may be occasionally useful in predicting the stages of HIV reactive individuals, especially in situations where the viral load and CD4 cell count results are not available.
| 1. |
Gurtler L. Difficulties and strategies of HIV diagnosis. Lancet 1996;348:176-9.
[Google Scholar]
|
| 2. |
Best SJ, Dax EM. Assays for HIV with improved sensitivity and specificity. Exp Opin Invest Drugs 1997;8:965-83.
[Google Scholar]
|
| 3. |
Centers for Disease Control. Provisional public health service inter-agency recommendations for screening donated blood and plasma for antibody to the virus causing acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep 1985;34:1-5.
[Google Scholar]
|
| 4. |
Centers for Disease Control. Public health service guidelines for counseling and antibody testing to prevent HIV infection and AIDS. MMWR Morb Mortal Wkly Rep 1987;36:509-15.
[Google Scholar]
|
| 5. |
Centers for Disease Control. Update: Serologic testing for antibody to human immunodeficiency virus. MMWR Morb Mortal Wkly Rep 1988;36:833-45.
[Google Scholar]
|
| 6. |
Baveja U. Antibody testing with special reference to HIV-1, Chapter 6. In : HIV testing manual, laboratory diagnosis, biosafety and quality control. National Institute of Communicable Diseases and National AIDS Control Organisation, Ministry of Health and Family Welfare, Govt. of India: New Delhi; p. 107-13.
[Google Scholar]
|
| 7. |
Instruction manual, Genelabs Diagnostics Pvt. Ltd. 85, Science Park, Drive #04-01, Singapore Science Park: Singapore; 118259.
[Google Scholar]
|
| 8. |
Instruction manual , J. Mitra and Co. Ltd.: New Delhi.
[Google Scholar]
|
| 9. |
Chattopadhya D, Aggarwal RK, Kumari S. Profile of antigen-specific antibody response detectable by Western blot in relation to diagnostic criteria for human immunodeficiency virus type-1 (HIV-1) infection. Clin Diagnost Virol 1996;7:35-42.
[Google Scholar]
|
| 10. |
Garland FC, Garland CF, Gorham ED, Brodine SK. Western blot banding patterns of HIV rapid progressors in the U.S. Navy Seropositive Cohort: Implications for vaccine development. Navy Retroviral Working Group. Ann Epidemiol 1996;6:341-7.
[Google Scholar]
|
| 11. |
Goudsmit J, Lange JM, Paul DA, Dawson GJ. Antigenemia and antibody titers to core and envelope antigens in AIDS, AIDS-related complex and subclinical human immunodeficiency virus infection. J Infect Dis 1987;155:558-60.
[Google Scholar]
|
Fulltext Views
9,394
PDF downloads
2,864





