Translate this page into:
18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: A randomized placebo-controlled pilot study
2 Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Nuclear Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
4 Department of Pediatrics Biochemistry, Postgraduate Institute of Medical Education and Research, Chandigarh, India
5 Department of Cardiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Samir Malhotra
Room No. 4035, Department of Pharmacology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Kaur S, Shafiq N, Dogra S, Mittal B R, Attri SV, Bahl A, Narang T, Vinay K, Rajagopalan S, Malhotra S. 18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: A randomized placebo-controlled pilot study. Indian J Dermatol Venereol Leprol 2018;84:660-666 |
Abstract
Background: Psoriasis is a systemic inflammatory disorder associated with an increased risk of cardiovascular disease.
Objective: To evaluate the utility of [[18]F]-fluorodeoxyglucose positron emission tomography/computed tomography in identifying vascular and systemic inflammation in psoriasis patients with moderate-to-severe disease and to analyze its usefulness in assessing the effect of systemic treatment.
Methods: This was a randomized, double-blind pilot study conducted in a tertiary care center. Baseline standardized uptake value score was estimated by18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with moderate-to-severe psoriasis and compared with historical controls. Patients were then randomized using computer-generated randomization list into methotrexate or placebo (with or without pioglitazone) groups.18F-fluorodeoxyglucose positron emission tomography/computed tomography was repeated at 12 weeks and composite standardized uptake value score determined. The correlation between Psoriasis Activity and Severity Index and SUVmax was assessed.
Results: A total of 16 patients were randomized to different treatment groups. Significant increase in mean SUVmax was observed in the ascending aorta in psoriasis patients as compared to historical controls (2.03 ± 0.53 vs 1.51 ± 0.36, P < 0.03). There was no difference in composite standardized uptake value score after 12 weeks of treatment in any of the treatment groups (P = 0.82), although an improvement in Psoriasis Activity and Severity Index score in the methotrexate arm was observed. No correlation was found between mean SUVmax and Psoriasis Activity and Severity Index scores in various aortic segments (r = 0.3–0.7).
Limitations: Small sample size, short follow-up, historical controls, exclusion of patients with comorbid conditions and lack of surrogate markers of systemic inflammation.
Conclusion: 18F-fluorodeoxyglucose positron emission tomography imaging showed higher vascular inflammation in ascending aorta of psoriasis patients as compared to historical controls. Systemic treatment with methotrexate and pioglitazone did not influence the vascular inflammation in the short term.
Introduction
Psoriasis is a chronic inflammatory disorder affecting 2–3% of the world population.[1] It is now considered as a systemic inflammatory disorder that is associated with metabolic syndrome, diabetes mellitus, nonalcoholic steatohepatitis, myocardial infarction, stroke and premature cardiovascular death.[2],[3] [18F]-Fluorodeoxyglucose positron emission tomography/computed tomography is a validated technique that enables highly precise, in vivo measurements of inflammatory activity including vascular, visceral and whole body inflammation. Recently, studies employing fluorodeoxyglucose positron emission tomography/computed tomography to evaluate areas of inflammation in psoriasis have reported higher risk of asymptomatic cardiac, joint and hepatic diseases.[4],[5],[6],[7],[8] The vascular inflammation detected by fluorodeoxyglucose positron emission tomography/computed tomography can be reversed by lifestyle modification, lipid-modifying drugs such as simvastatin and use of antipsoriatic medications, chiefly tumor necrosis factor-alpha inhibitors.[9],[10],[11] However, studies evaluating the effect of conventional antipsoriatic medications on systemic/vascular inflammation are few in number.[6] Methotrexate is a known antiinflammatory agent and acts by increasing adenosine efflux.[12] We have earlier demonstrated that pioglitazone, an insulin sensitizer, results in a reduction in Psoriasis Activity and Severity Index score.[13],[14]
We conducted this study with a primary aim of evaluating fluorodeoxyglucose positron emission tomography/computed tomography as a potential imaging biomarker for detection of vascular and systemic inflammation in patients of moderate to severe psoriasis. The secondary aims were to determine the effect of methotrexate and pioglitazone on systemic inflammation and Psoriasis Activity and Severity Index score.
Methods
Study design and patients
This was a prospective, single-center, factorial design, randomized, placebo-controlled blinded pilot study in patients with moderate-to-severe psoriasis (CTRI/2014/08/004842). All psoriasis patients attending the psoriasis clinic of Postgraduate Institute of Medical Education and Research, Chandigarh from March 2013 to December 2013 were screened for inclusion in this study. Patients satisfying the inclusion and exclusion criteria and consenting for study were recruited. Age and sex-matched historical controls were taken from patients who underwent positron emission tomography/computed tomography imaging for suspected malignancy but were found to have no pathological anomaly. The study was conducted in accordance with the principles of Declaration of Helsinki and was approved by the Ethics Committee of our institute. The overall study design is depicted in [Figure - 1].
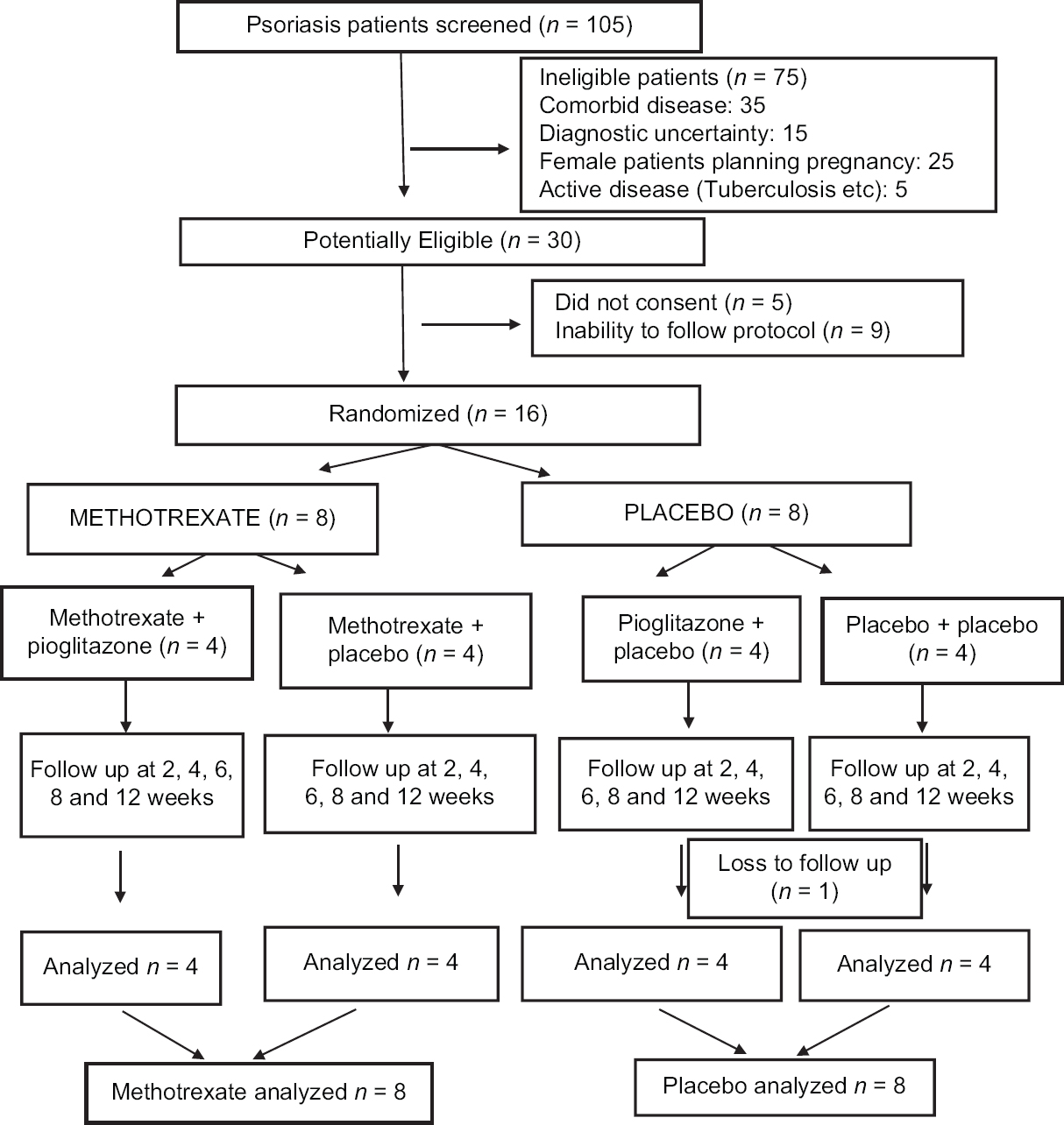 |
| Figure 1: Study design |
Inclusion criteria werepatients with moderate and severe psoriasis (Psoriasis Activity and Severity Index ≥5) of age range 18–70 years. The exclusion criteria were: (a) chronic inflammatory diseases such as diabetes mellitus, uncontrolled hypertension (>180 mmHg/95 mmHg), cardiovascular diseases, liver disease and psoriatic arthritis; (b) systemic therapy for psoriasis in preceding 3 months; (c) smoking/alcohol use (>2 drinks per day); (d) intravenous drug use; and (e) pregnancy or lactation.
Baseline evaluation
Detailed demographic and disease data was recorded for each patient. Baseline disease severity was assessed by Psoriasis Activity and Severity Index score.[15] Complete blood counts, liver and renal function tests and positron emission tomography/computed tomography imaging were done in all recruited patients at baseline.
Positron emission tomography/computed tomography methodology
Fluorodeoxyglucose positron emission tomography/computed tomography was done in all recruited patients and standardized uptake value (SUVmax) was used as a measure of fluorodeoxyglucose uptake.18F-fluorodeoxyglucose positron emission tomography/computed tomography scan was performed using a Discovery LS (GE) positron emission tomography scanner. 10mCi of 18F-fluorodeoxyglucose was injected intravenously after 6 h of fasting. Total body images (from head to toe with hands by the side) were acquired in three-dimensional mode 60 min after intravenous injection of 370 MBq of 18F-fluorodeoxyglucose using a dedicated positron emission tomography/computed tomography scanner. Reconstruction of the acquired data was performed so as to obtain fused positron emission tomography/computed tomography images in transaxial, coronal and sagittal views. From the raw emission data collected, the image was reconstructed by iterative reconstruction with computed tomography-derived attenuation correction using the ordered subsets expectation maximization algorithm.
Treatment and follow-up
All recruited patients were randomized to receive either methotrexate once a week or placebo. Due to the wide variation in the age and body weight, a weight-based dosage of 0.5 mg/kg/week of methotrexate was chosen over a fixed-dose regimen.[16] All recruited patients also received folic acid supplementation at a dose of 2.5 mg 2 days/week. Methotrexate and placebo groups were further subdivided into two subgroups, with each receiving either pioglitazone 30 mg/day or placebo. [Figure - 1]. Thus, there were four subgroups, Methotrexate with pioglitazone, methotrexate with placebo, placebo with pioglitazone and placebo with placebo. Though subgrouping led to a small sample size in each group, the purpose of this pilot study was to gather information for designing adequately powered study later with a single-focused question.
In the current study, computer-generated randomization list was used to assign patients to different treatment groups. Randomization list was generated by a pharmacist and kept in sealed envelopes. The allocation group of the eligible patients was revealed only at the time of drug dispensation. Patients were clinically evaluated at weeks 2, 4, 6, 8 and 12. Fluorodeoxyglucose positron emission tomography/computed tomography was repeated at the final visit.
Study outcomes
The primary outcome was to compare the baseline SUVmax of psoriasis patients with historical controls. Secondary outcomes included (1) change in the composite standardized uptake value score [mean of SUVmax of all segments of aorta (ascending aorta, arch of aorta, descending aorta, suprarenal aorta, infrarenal aorta) liver, skin and joints] at baseline and after 12 weeks of treatment with methotrexate or placebo in study subjects; (2) assessment of effect of treatment on the individual components of mean SUVmax score; (3) effect of adding pioglitazone on composite standardized uptake value score; (4) correlation between positron emission tomography/computed tomography findings and Psoriasis Activity and Severity Index score.
Safety reporting
Safety data was obtained by patient interview and examination at all study visits and by doing laboratory investigations such as complete blood count, renal and liver function tests at 0, 2, 4, 8 and 12 weeks. All adverse drug events were monitored at each postrandomization visit by means of open and closed questions to the study participants. Patients developing any of the following criteria were withdrawn from the study: (a) disease exacerbation (increase in Psoriasis Activity and Severity Index >25% of baseline score), (b) any serious/severe adverse drug event warranting cessation of treatment.
Sample size calculation
For the primary outcome, assuming standard deviation of 6 in Psoriasis Activity and Severity Index scores, and a difference of 12 between drug and placebo arm at 12 weeks to be clinically significant in Psoriasis Activity and Severity Index score (α = 0.05, 80% power), a sample size of six patients per group had been calculated. Keeping in mind a dropout rate of 20%, eight patients were required to be included in each group. This sample size gives us about 70% power to detect a difference of 0.16 SUV between two groups assuming a standard deviation of SUV of 0.15 based on previous observation.[4]
Statistical analysis
Descriptive data were expressed in mean ± standard deviation. Total composite score was evaluated by taking means of SUVmax of all segments of aorta combined with liver, skin and joints. Aortic composite score was evaluated by taking means of SUVmax of all aortic segments (ascending aorta, arch of aorta, descending aorta, suprarenal aorta and infrarenal aorta). Change in composite score of SUVmax from baseline was analyzed using univariate analysis of variance. Change in individual score from baseline was analyzed by Wilcoxon ranked-sum test and between group differences were analyzed by Kruskal–Wallis method. Multivariate analysis was used to evaluate change in composite score among different groups. Categorical data were analyzed by Chi-square or Fischer's exact test. All patients who received at least one dose of study drug were included in the safety analysis. Intention to treat analysis was used. Missing values were handled using multiple imputation.
Results
Patient characteristics
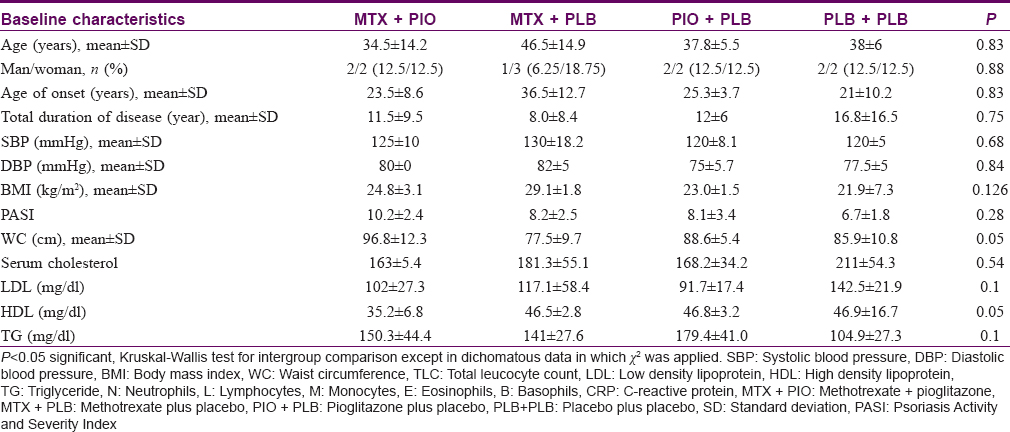
Out of the 105 psoriasis patients screened, 30 patients were potentially eligible for this study. Out of these 30 patients, 5 did not give consent, and 9 patients had limitations for follow-up and hence were excluded. A total of 16 patients were randomized to methotrexate (n = 8) or placebo (n = 8). They were further randomized to four treatment groups: methotrexate + pioglitazone, methotrexate + placebo, pioglitazone + placebo and placebo + placebo [Figure - 1]. At baseline, there was no significant difference in the demographic characteristics between four treatment groups with respect to various disease parameters, demographic profile and mean SUVmax [Table - 1]. Fifteen patients completed the follow-up duration of 12 weeks. One patient from pioglitazone + placebo arm dropped out after 4 weeks of treatment because of noncompliance.
Primary outcome
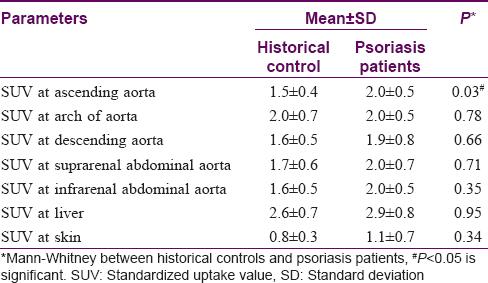
On comparing SUVmax of psoriasis patients with historical controls, a significant difference was observed in mean SUVmax (higher value) in the ascending aorta of psoriasis patients (2.0 ± 0.5) as compared to historical controls (1.5 ± 0.4); P = 0.03 [Table - 2]. None of the other individual SUVmax showed any statistically significant difference [Table - 2].
Secondary outcomes
The mean SUVmax values in different groups at 12 weeks ranged from 0.5 ± 0.3 to 3.5 ± 1.0 [Table - 3]. There was no significant difference in composite SUVmax score after 12 weeks of treatment in patients randomized to methotrexate or placebo (P = 0.825). Even after applying imputation methods (for missing data), no significant change was observed in SUVmax. There was no significant difference in the SUVmax in other segments of aorta, liver, joints and even skin between the psoriasis patients and controls [Table - 3]. There was no significant difference among four treatment groups in SUVmax. No difference was observed after adding pioglitazone to methotrexate in the composite score for SUV.
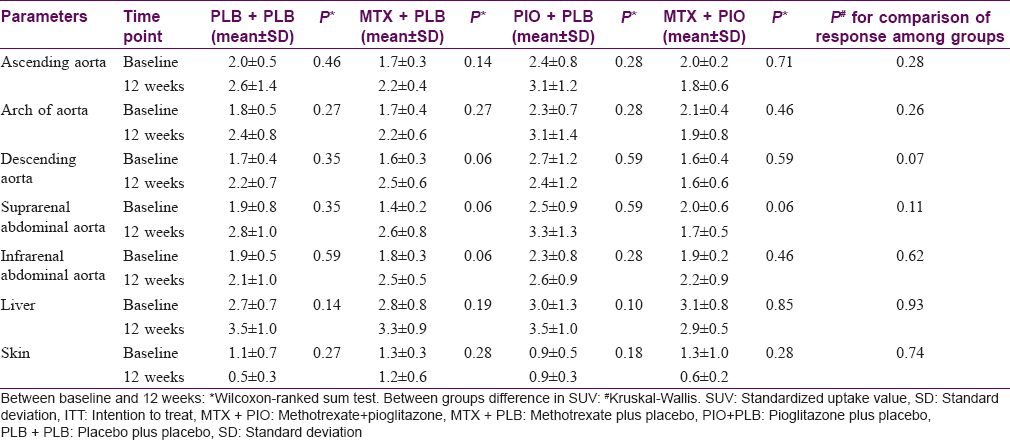
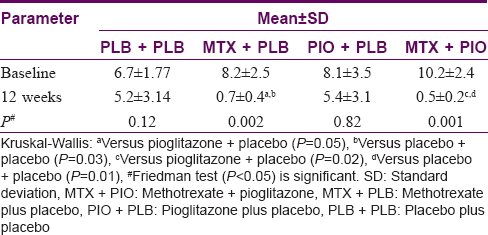
There was a statistically significant difference in Psoriasis Activity and Severity Index scores from baseline to 12 weeks in methotrexate + pioglitazone and methotrexate + placebo group. In methotrexate + pioglitazone, Psoriasis Activity and Severity Index score decreased from 10.2 ± 2.4 to 1.2 ± 0.8; P = 0.002 and in methotrexate + placebo group from 8.1 ± 3.5 to 5.4 ± 3.1; P = 0.001 [Table - 4]. No correlation was found between change in Psoriasis Activity and Severity Index score and change in SUVmax in psoriasis patients from baseline to 12 weeks in any of the four treatment groups (r = 0.3 -0.47).
Safety evaluation
In methotrexate group, adverse events observed were cheilitis (n = 1), followed by nausea (n = 1), vomiting (n = 1) and dizziness (n = 1). In pioglitazone group, weight gain of >2 kg was observed in two patients. All adverse events were mild in nature and did not require treatment discontinuation.
Discussion
Fluorodeoxyglucose positron emission tomography/computed tomography is a sensitive and noninvasive imaging modality for detecting cellular inflammation and has been recently used to demonstrate subclinical inflammation of blood vessels in patients with psoriasis.[4],[5],[6],[7],[8] Early detection of these changes in vessels can help in initiation of preventive measures or interventions in the management of both psoriasis and vascular inflammation.
We observed that mean SUVmax in ascending aorta in psoriasis patients was higher as compared to control group. These findings were similar to earlier studies in patients with moderate-to-severe psoriasis, which showed increased vascular inflammation in ascending, descending and infrarenal aorta.[4],[5] Recently, Youn et al. have reported similar findings even in patients with mild psoriasis.[7] Khalid et al. reported an increased association between psoriasis and aortic stenosis. The authors attributed this to the inflammation in aortic valve and ascending aorta.[17] Increased aortic inflammation has also been reported in psoriasis patients with sacroilitis.[18]
Other target sites such as descending aorta, liver, joints and skin did not show any significant differences in SUVmax when compared to historical controls. In our patients, the mean SUVmax at baseline (aortic segments, liver, skin, joints) ranged from 0.6 ± 0.5 to 4.1 ± 3.2, which is not indicative of high degree of inflammation. In earlier studies evaluating vascular and systemic inflammation (aorta, liver, skin, musculoskeletal areas), the SUVmax has ranged from 0.4 ± 0.2 to 2.1 ± 0.2.[4],[7],[8] The authors in these studies have considered it to be indicative of inflammation. However, the use of these cutoff values for inflammation is debatable.[4],[5],[6],[7] Use of surrogate markers of systemic inflammation, such as C-reactive protein, tumor necrosis factor and interleukin-6, could help in clarifying this inconsistency and should be assessed in future studies. Because there was no evidence of inflammation (SUVmax) at baseline in these psoriatic patients, no statistically significant change was observed in composite scores in methotrexate and placebo groups after 12 weeks of treatment.
A systematic review and meta-analysis of observational studies evaluating an association between methotrexate and cardiovascular diseases in patients with rheumatoid arthritis, psoriasis and polyarthritis showed a 21% lower risk for total cardiovascular diseases [n = 10 studies, 95% confidence interval (0.73 to 0.87), P < 0.001] and an 18% lower risk for myocardial infarction [n = 5, 95% confidence interval (0.71 to 0.96), P = 0.01].[19] All studies were adjusted for underlying cardiovascular diseases risk factors such as smoking, cholesterol and blood pressure. This implicates that methotrexate is effective not only as antipsoriatic and antirheumatic but also curtails cardiovascular diseases mortality. Our study excluded patients with associated cardiovascular diseases risk factors such as uncontrolled hypertension, hypercholesterolemia, diabetes and chronic smokers; hence to evaluate its effect on cardiovascular diseases, we need long-term studies.
Mehta suggested that fluorodeoxyglucose positron emission tomography/computed tomography can be used as a biomarker in a dynamic disease such as psoriasis as it helps to detect underlying inflammation linking chronic inflammatory disease states such as atherosclerosis, metabolic syndrome, diabetes mellitus and psoriatic arthritis with psoriasis.[4] However, for a biomarker to be associated with a condition, the treatment, which brings about improvement in clinical condition should demonstrate a similar trend in the proposed biomarker. In our study, this trend was not noted. A significant decrease in Psoriasis Activity and Severity Index scores was observed in the methotrexate group but the decline in SUVmax values failed to reach statistical significance. The fall in Psoriasis Activity and Severity Index did not correlate with change in mean SUVmax. Further adding pioglitazone to either methotrexate or placebo did not show any difference in SUVmax, suggesting no effect of pioglitazone on SUVmax.
The results of the current study differed from our previous studies wherein a meta-analysis conducted on pioglitazone shows a significant decrease in Psoriasis Activity and Severity Index score in psoriasis patients.[13],[14],[20] This may be due to the exclusion of patients with underlying hypertension, diabetes, dyslipidemia, active smoking, coronary atherosclerosis and psoriatic arthritis to avoid any confounding variables.
Although the values of SUVmax in psoriasis patients in our study were higher than those found in previous studies, there was no statistically significant difference when compared to controls except for increased uptake in ascending aorta.[4],[5] This could be because of several reasons that affect standardized uptake value, which include body size, body weight, respiratory motion, post injection uptake time and blood glucose levels. Technical factors affecting SUVmax include scanner variability and timing mismatch. These factors need to be looked after while taking into account standardized uptake value.[21] A double-blind controlled study by Bissonnette et al. showed a reduction in vascular inflammation in moderate-to-severe psoriasis patients by adalimumab, but the difference was not large enough to be demonstrated in a study with a small sample size.[6] However, the parameter used for assessment of inflammation was total background ratio, which is postulated to be of high reliability as compared to SUVmax in detecting inflammation in one of the study, which could possibly explain for differences observed by us.[22]
Limitations of our study were small sample size, short follow-up, historical controls, exclusion of patients with comorbid conditions and lack of surrogate markers of systemic inflammation such as C-reactive protein, tumor necrosis factor and interleukin-6.
Conclusion
In conclusion, we observed higher vascular inflammation in ascending aorta of psoriasis patients as compared to controls. Systemic treatment with methotrexate and pioglitazone did not influence the vascular inflammation in the short term. For definitive conclusion, large randomized studies with adequate sample size and longer follow-up are required. This would also help in evaluation of effect of different treatment modalities on SUVmax, thus maximizing our understanding of arterial wall inflammation, and its relationship to psoriasis severity, response to treatment and future cardiovascular events. Further, assessment of performance characteristics, reproducibility and accuracy for the validity of 18F-fluorodeoxyglucose positron emission tomography/computed tomography needs to be elucidated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496-509.
[Google Scholar]
|
| 2. |
Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: A prospective study of US female nurses. Arch Dermatol 2009;145:379-82.
[Google Scholar]
|
| 3. |
Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol 2008;159:895-902.
[Google Scholar]
|
| 4. |
Mehta NN. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography – Computed tomography (FDG-PET/CT). Arch Dermatol 2011;147:1031.
[Google Scholar]
|
| 5. |
Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: A pilot study. Am J Cardiovasc Dis 2013;3:273-8.
[Google Scholar]
|
| 6. |
Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC, et al. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: Results of a randomized controlled trial. Circ Cardiovasc Imaging 2013;6:83-90.
[Google Scholar]
|
| 7. |
Youn SW, Kang SY, Kim SA, Park GY, Lee WW. Subclinical systemic and vascular inflammation detected by (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with mild psoriasis. J Dermatol 2015;42:559-66.
[Google Scholar]
|
| 8. |
Hjuler KF, Gormsen LC, Vendelbo MH, Egeberg A, Nielsen J, Iversen L, et al. Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis. Br J Dermatol 2017;176:732-40.
[Google Scholar]
|
| 9. |
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825-31.
[Google Scholar]
|
| 10. |
Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH, et al. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008;49:1277-82.
[Google Scholar]
|
| 11. |
Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: A case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol 2017;2:1013-8.
[Google Scholar]
|
| 12. |
Glossmann H, Reider N. A marriage of two “Methusalem” drugs for the treatment of psoriasis?: Arguments for a pilot trial with metformin as add-on for methotrexate. Dermatoendocrinol 2013;5:252-63.
[Google Scholar]
|
| 13. |
Shafiq N, Malhotra S, Pandhi P, Gupta M, Kumar B, Sandhu K, et al. Pilot trial: Pioglitazone versus placebo in patients with plaque psoriasis (the P6). Int J Dermatol 2005;44:328-33.
[Google Scholar]
|
| 14. |
Mittal R, Malhotra S, Pandhi P, Kaur I, Dogra S. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: A randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol 2009;145:387-93.
[Google Scholar]
|
| 15. |
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50.
[Google Scholar]
|
| 16. |
Menting SP, Dekker PM, Limpens J, Hooft L, Spuls PI. Methotrexate dosing regimen for plaque-type psoriasis: A systematic review of the use of test-dose, start-dose, dosing scheme, dose adjustments, maximum dose and folic acid supplementation. Acta Derm Venereol 2016;96:23-8.
[Google Scholar]
|
| 17. |
Khalid U, Ahlehoff O, Gislason GH, Skov L, Torp-Pedersen C, Hansen PR, et al. Increased risk of aortic valve stenosis in patients with psoriasis: A nationwide cohort study. Eur Heart J 2015;36:2177-83.
[Google Scholar]
|
| 18. |
Rose S, Dave J, Millo C, Naik HB, Siegel EL, Mehta NN, et al. Psoriatic arthritis and sacroiliitis are associated with increased vascular inflammation by 18-fluorodeoxyglucose positron emission tomography computed tomography: Baseline report from the psoriasis atherosclerosis and cardiometabolic disease initiative. Arthritis Res Ther 2014;16:R161.
[Google Scholar]
|
| 19. |
Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol 2011;108:1362-70.
[Google Scholar]
|
| 20. |
Malhotra A, Shafiq N, Rajagopalan S, Dogra S, Malhotra S. Thiazolidinediones for plaque psoriasis: A systematic review and meta-analysis. Evid Based Med 2012;17:171-6.
[Google Scholar]
|
| 21. |
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010;195:310-20.
[Google Scholar]
|
| 22. |
Niccoli Asabella A, Ciccone MM, Cortese F, Scicchitano P, Gesualdo M, Zito A, et al. Higher reliability of 18F-FDG target background ratio compared to standardized uptake value in vulnerable carotid plaque detection: A pilot study. Ann Nucl Med 2014;28:571-9.
[Google Scholar]
|
Fulltext Views
3,367
PDF downloads
2,365





