Translate this page into:
Imatinib mesylate induced erythroderma
Correspondence Address:
Swapnil A Sanghavi
Department of Skin and VD, Seth G.S. Medical College and KEM Hospital, Parel, Mumbai 400 012, Maharashtra
India
| How to cite this article: Sanghavi SA, Dongre AM, Khopkar US. Imatinib mesylate induced erythroderma. Indian J Dermatol Venereol Leprol 2012;78:408 |
Sir,
Imatinib is the first molecularly targeted drug developed for the treatment of Chronic myeloid leukemia (CML) and has achieved remarkable success. It is a tyrosine kinase inhibitor, which is extensively used for treatment of malignancies. Here, we report a case of erythroderma induced by imatinib in a case of CML, which was severe enough to warrant discontinuation of the therapy. Imatinib-induced erythroderma is uncommon in occurrence and only a few reports including one from India have been mentioned in literature. [1]
A 36-year-old woman presented with complaints of redness and itching all over the body, including the face, of the duration of five days. She was a known case of CML diagnosed in 2006 and was treated with hydroxyurea. Her recent drug history revealed that she was on oral imatinib Mesylate 800 mg/day and her skin rash started two days after the initiation of imatinib. Along with skin rash she had low-grade fever of the duration of four days. On further probing, she gave a history of similar type of skin rash 3 months back, which started about 15 days after starting treatment with imatinib; after this, imatinib was discontinued by the hematologist. Following this, the rash subsided completely in one month. The drug was recently restarted at a dose of 800 mg/day following which she redeveloped similar rash with greater severity.
On examination, diffuse erythema with fine scaling was observed all over the body including palm, soles, and face [Figure - 1]. Erythema and scaling were more pronounced over the trunk [Figure - 2] and periorbital region. Her fingernails were hyperpigmented. No significant lymphadenopathy or hepatosplenomegaly was observed in the patient.
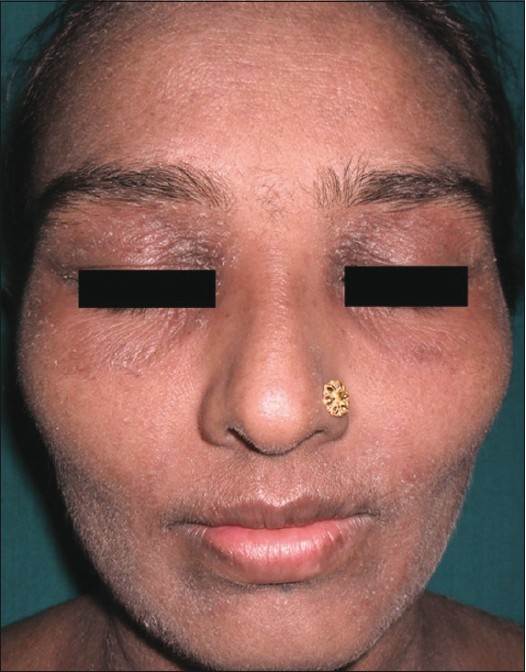 |
| Figure 1: Erythema and scaling on the face |
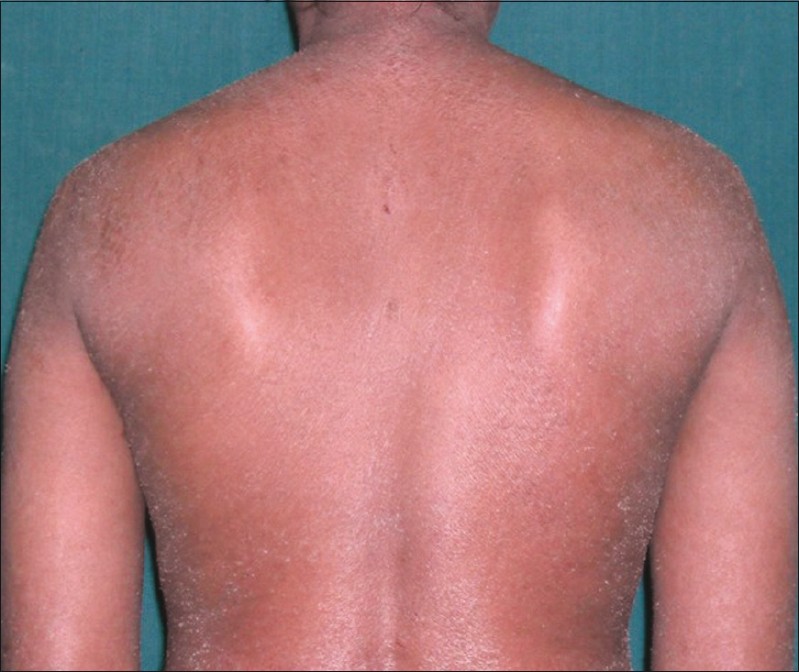 |
| Figure 2: Erythroderma over the trunk and extremities |
A skin biopsy done from back showed epidermal hyperplasia, foci of parakeratosis, spongiosis, and a few necrotic keratinocytes [Figure - 3]. Dermis showed sparse superficial perivascular infiltrate consisting of lymphocytes and few eosinophils. Her liver function tests, total blood counts including eosinophil count (2%) and renal function tests showed normal results.
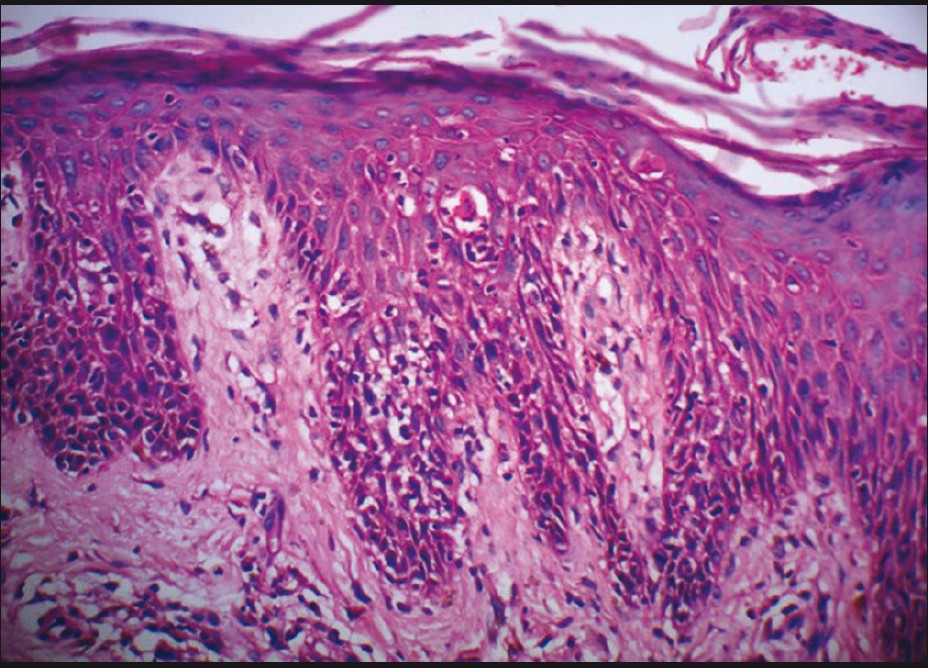 |
| Figure 3: Epidermal hyperplasia with parakeratosis, spongiosis, and necrotic keratinocytes (H and E, ×200) |
Imatinib was discontinued. She was started on oral antihistaminics and oral prednisolone 40 mg/day. The rash subsided over a period of 7 days [Figure - 4]. Avoidance of imatinib mesylate was advised and the hematologists are now planning to start her on Dasatinib.
Imatinib acts by inhibition of several tyrosine kinase enzymes, including Bcr-Abl tyrosine kinase. [1] It is the initial choice of treatment for most patients with CML.
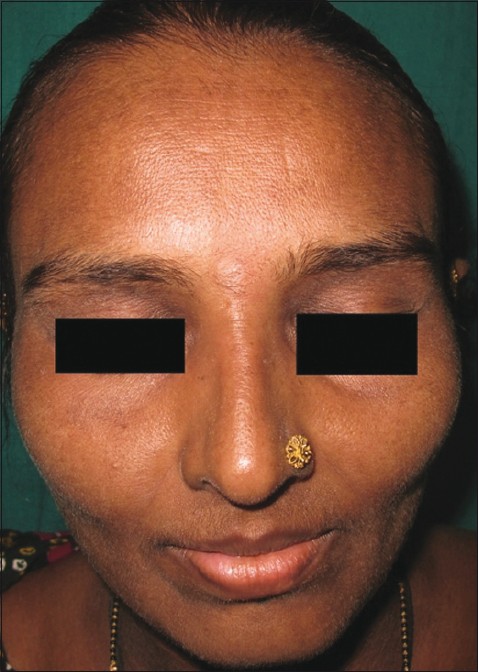 |
| Figure 4: Erythema and scaling disappeared 7 days after discontinuation of imatinib |
Imatinib has been commonly known to cause non-dermatological side effects. The reported incidence of cutaneous reactions to imatinib varies from 9.5% to 69%. [2] Milder skin reactions are more common (30-40%) than the severe ones (2-5%). The drug acts as a dose-dependent inducer of cutaneous adverse reactions with mild reactivity to low or intermediate doses (200-600 mg/day), but severe eruptions to high dose (600-1000 mg/day). The common dermatological side effects include maculopapular eruptions and periorbital edema, which are usually self-limited. Severe cutaneous adverse effects such as Stevens Johnson′s syndrome, toxic epidermal necrolysis, and acute generalized exanthematous pustulosis are uncommon in occurrence. Rare cutaneous eruptions are exfoliative dermatitis, lichenoid reactions, pityriasiform eruptions, psoriasis, and reactivation or induction of porphyria cutanea tarda. [2] Re-pigmentation of gray hair, hyperpigmentation of the nails, [3] and skin hypopigmentation have been reported.
Our extensive literature search revealed only a few case reports of imatinib-induced exfoliative dermatitis. Imatinib-induced exfoliative dermatitis usually occurs 1-3 weeks following initial exposure and within few hours to few days following a re-challenge. The exact mechanism of drug reaction is not known, but occurrence of eruption immediately after re-challenge suggest hypersensitivity rather than a pharmacological effect. [4]
In case of mild cutaneous adverse effects, imatinib need not be discontinued; such cases can be managed with antihistaminics and topical steroids. In some patients, a strategy of initial discontinuation of imatinib followed by restart with or without topical/systemic steroids can be useful. Severe adverse reactions necessitate discontinuation of the drug. Oral desensitization has been found to be successful in some patients with leukemia. [4] For imatinib-intolerant patients, dasatinib and nilotinib are the alternatives. [5]
Overall, the incidence of skin rash due to dasatinib and nilotinib is low in comparison with that due to imatinib. The reported incidence of maculopapular rash is ≤34% and 20% in patients on imatinib and nilotinib or dasatinib, respectively, whereas that of severe skin rash is 2% with imatinib therapy as compared to <1% with dasatinib and nilotinib. The incidence of cross-reactivity between imatinib and other tyrosine kinase inhibitors is very low. [5]
Thus, we report a rare case of imatinib-induced erythroderma, which required cessation of therapy. Dermatologists should be aware of imatinib as a cause of drug-induced erythroderma.
| 1. |
Mathew T, Chandrashekar L, Pulimood S, Srivastava A. Imatinib induced erythroderma. Australas J Dermatol 2007;48:193-4.
[Google Scholar]
|
| 2. |
Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest 2000;105:3-7.
[Google Scholar]
|
| 3. |
Prabhash K, Biswas G, Prasad N, Karant N, Sastry PS, Parikh PM. Imatinib-induced nail hyperpigmentation in chronic myeloid leukemia. Indian J Dermatol Venereol Leprol 2006;72:63-4.
[Google Scholar]
|
| 4. |
Nelson RP Jr, Cornetta K, Ward KE, Ramanuja S, Fausel C, Cripe LD. Desensitization to imatinib in patients with leukemia. Ann Allergy Asthma Immunol 2006;97:216-22.
[Google Scholar]
|
| 5. |
Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - A review on pharmacology, metabolism and side effects. Curr Drug Metab 2009;10:470-81.
[Google Scholar]
|
Fulltext Views
2,352
PDF downloads
2,085





