Translate this page into:
Skin detoxification cycles
2 Facultad de Ciencias Qu�micas, CIB-Doctorado en Ciencias Biom�dicas, Universidad Veracruzana, Mexico
3 Escuela M�dico Militar, UDEFA, DF, Mexico
Correspondence Address:
Ismael V�squez-Moctezuma
Maestr�a en Ciencias de la salud, ESM-IPN
Mexico
| How to cite this article: V�squez-Moctezuma I, M�ndez-Bolaina E, S�nchez-Gonz�lez DJ. Skin detoxification cycles. Indian J Dermatol Venereol Leprol 2012;78:414-416 |
Melanocytes have until very recently been classified as part of a protective mechanism, with melanin shielding the organism from radiation emitted by type B ultraviolet light. New findings about the regulation and distribution of melanocytes, implying heterogeneity of production and transfer of pigments to different cutaneous regions, lead to the conclusion that the function of these cells is multifaceted and of profound importance. [1]
The stratified squamous epithelium is complex, with abundant intercellular contacts that include homotropic as well as heterotrophic communication between keratinocytes and melanocytes. We propose that the melanocyte has the potential of detoxifying the skin, utilizing antioxidant mechanisms and molecules that confer the multidrug resistance (MDR) phenotype, such as p-glycoprotein ABCB5, as detoxification pumps. If true, this would help to explain the presence of melanocytes in the palmoplantar skin, as well as the chemo-resistance of the melanoma. [1],[2],[3]
We suggest a model that would explain how genotoxic substances that enter the epidermis, whether from the external environment or the bloodstream, are eliminated from this skin layer by normal or malignant melanocytes. According to this model, genotoxic substances are scavenged by melanosomes and transported to keratinocytes, where they are eliminated as senescent cells. In the case of melanoma, the melanosomes would be transferred to the cells that form the tumoral microenvironment in such a way that they establish cycles of detoxification.
A melanocyte is one of the most complex and specialized cells, evidenced by the fact that its dendritic morphology reaches the membrane of 35 to 40 keratinocytes (the melano-epidermic unit). This cell transfers mature melanosomes by filopodia to the cytoplasm of the target cell. This is an active process that is modified with hormones and type B ultraviolet radiation. Apart from the fact that the melano-epidermic unit does not remain stable, the dendrites from the melanocyte actively connect and disconnect with the keratinocytes. This mechanism has come to be considered a type of epidermic circulation. [3]
There are marked differences between palmoplantar and other skin tissues. The concentration of melanocytes is five-fold less in the palmoplantar areas than in the rest of skin tissue. Yamaguchi et al. have found evidence of the genes and signaling pathways that control the development and maintenance of the phenotypical characteristics of palmo-plantar skin. They detected that the product of the DKK gene, synthesized by fibroblasts in palmoplantar skin, diminish the function, synthesis and transfer of melanin of the melanocytes of these cutaneous regions. [4] These findings lend themselves to the conclusion that the primordial role of melanocytes of palmoplantar skin should be different than, or possibly have an additional function to such cells found in pigmented skin.
In the past decade, many insights have been made into melanocyte biology. It is known that these cells are capable of expressing the ABCB5 gene, which codifies for the p-glycoprotein. This protein has a multidrug resistance phenotype, acting as an MDR pump, an important characteristic in the resistance of melanoma to chemotherapy. This pump has been detected in the plasma membrane of normal melanocytes, and shows increased function in malignant melanocytes. Chen et al. proposed a model of the scavenging of drugs by the p-glycoprotein ABCB5, which is located in the membrane of melanosomes. This mechanism can explain in part the chemo-resistance of malignant melanoma. [2],[5]
There is increasing evidence that melanocytes have more functions than those traditionally ascribed to it. For instance, it has been suggested that these cells process and present antigens in a way similar to dendritic cells. In this same sense, there are reports that identify and purify RNA in mature melanosomes of the hamster melanoma. [6] These ribonucleic acids could be transferred to keratinocytes and to cells of the tumoral microenvironment, where they might then carry out their function, whether by translating for or forming part of the families of microRNAs regulators of gene expression [Figure - 1]a and b.
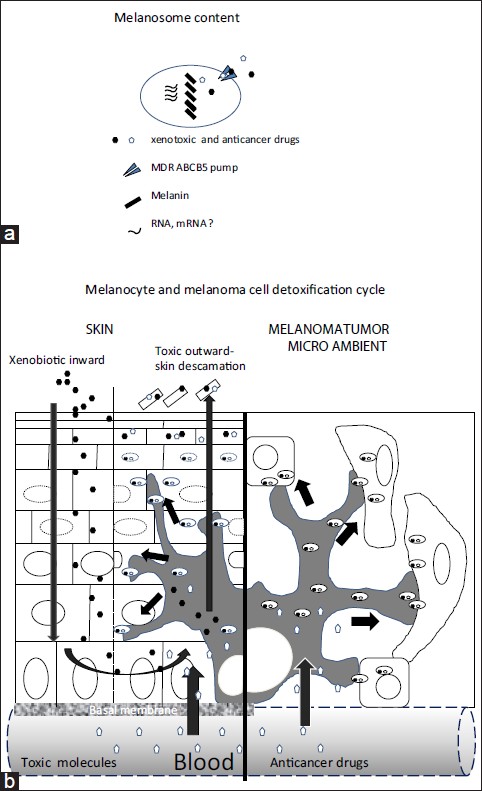 |
| Figure 1: Proposed model (a) Melanosome content: the melanocyte could transfer melanin as well as non-pigment molecules, the latter including messenger RNA (b) Detoxification: the entrance of genotoxic agents to the skin, whether from the environment or the bloodstream, activates cycles of detoxification involving the MDR ABCB5 pump, in which this agent enters the melanocyte, then is packaged in the melanosome and tranfered to keratinocytes. Thus during chemotherapy, in the microenvironment of the melanoma tumor, the malignant melanocyte could transfer the molecules of the cancer therapy to neighboring cells (such a fibroblasts) by means of the MDR ABCB5 pump that is harbored in the membrane the melanosomes |
Evolutionarily speaking, the palmoplantar skin, together with the lips and oral mucosa, are the areas of the epithelium most exposed to constant friction as well as contact with genotoxic molecules from the environment. Early man touched and grabbed leaves, fruit, roots, and branches of unknown plants whose substances could have been toxic. The palmoplantar skin, like the mucosa, should be adapted to protect against damage and invasion by substances toxic to the host. Indeed, hyperpigmentation is a frequent occurrence after the application of alkylating agents or anticancer antibiotics. [7] This hyperpigmentation would explain the mechanisms of detoxification orchestrated by melanocytes that attempt to eliminate and inactivate these agents by scavenging them in the mature melanosome and transferring them to the cytoplasm of the keratinocyte, an action coordinated by MDR pumps and antioxidant mechanisms [Figure - 1]a and b.
Fusogenic Property of The P-Glycoprotein Abcb5 And Detoxification Cycles
Frank et al. predicted the sequence of the p-glycoprotein ABCB5 upon analyzing the gene and the aminoacid through in vitro experiments. [2] This research group also deduced the MDR as well as fusogenic properties of this p-glycoprotein. Recently Yang et al. studied the role of cellular fusion and chemo-resistance with the MCF-7 breast cancer cell line, finding that the p-glycoprotein is involved in the heterogeneity of these cells and their chemo-resistance to doxorubicin. [8] Thus the ABCB5 pump must give the benign and malignant melanocyte an advantage in fissioning with fibroblasts, keratinocytes and other cellular lineages. [2],[8]
The malignant melanocyte camouflages itself and subjugates neighboring normal cells, and in this way establishes an adequate microenvironment that gradually extends itself. The p-glycoprotein ABCB5 is crucial in this process of camouflage and subjugation of neighboring cells, and therefore for the malignancy of cancer. As part of this process, malignant melanocytes transfer melanosomes loaded with genotoxic molecules (such as from chemotherapy) to neighboring cells, which are converted into a type of scavenger cell. [2],[8]
In conclusion, we propose a cutaneous model of the scavenging and elimination of genotoxic substances, with the participation of antioxidant mechanisms and MDR pumps, which establish detoxification cycles. This detoxification function of melanocytes must be evolutionarily more evident in palmoplantar skin, as it has an inseparable relationship with toxic molecules from the environment. For the epidermis, this detoxification mechanism is orchestrated by the melano-epidermic unit and is carried out through MDR transporters. This cycle, according to our model, would begin by the entrance of toxic substances from the environment or the bloodstream to the basal epidermis, substances which pass through different skin layers until being scavenged by melanocytes. These toxic substances would then be incorporated into mature melanosomes and transported by means of the dendritic trees to the cytoplasm of keratinocytes, to finally be eliminated by skin flaking of senescent cells. Malignant melanocytes would utilize a similar mechanism, with some variation.
Since it is known that melanocytes of other tissues, such as the epithelium of the retina and internal ear, participate in the development, maintenance and detoxification of these organs, it is likely that pigmentary cells in the skin are involved in complex functions of detoxification and maintenance as well. If this is true, melanocytes in the skin would have to coordinate themselves with cytochrome and MDR proteins associated with the MDR of the keratinocytes in the epidermis as well as the specialized structures like the hair follicle.
| 1. |
Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, et al. What are melanocytes really doing all day long...? Exp Dermatol 2009;18:799-819.
[Google Scholar]
|
| 2. |
Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 p-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem 2003;278:47156-65.
[Google Scholar]
|
| 3. |
Singh SK, Kurfurst R, Nizard C, Schnebert S, Perrier E, Tobin DJ. Melanin transfer in human skin cells is mediated by filopodia-a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J 2010;24:3756-69.
[Google Scholar]
|
| 4. |
Yamaguchi Y, Passeron T, Watabe H, Yasumoto K, Rouzaud F, Hoashi T, et al. The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: Mechanisms underlying its suppression of melanocyte function and proliferation. J Invest Dermatol 2007;127:1217-25.
[Google Scholar]
|
| 5. |
Chen KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res 2009;22:740-9.
[Google Scholar]
|
| 6. |
Vedralová E, Duchon J, Hach P. RNA and DNA in melanosomes of hamster melanoma. Pigment Cell Res 1987;1:76-80.
[Google Scholar]
|
| 7. |
Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol 1999;40:367-98.
[Google Scholar]
|
| 8. |
Yang JY, Ha SA, Yang YS, Kim JW. P-Glycoprotein ABCB5 and YB-1 expression plays a role in increased heterogeneity of breast cancer cells: Correlations with cell fusion and doxorubicin resistance. BMC Cancer 2010;10:388.
[Google Scholar]
|
Fulltext Views
3,853
PDF downloads
1,739





