Translate this page into:
Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results
2 Department of Dermatology, Venereology and Leprosy, RPGMC, Kangra (Tanda), Himachal Pradesh, India
Correspondence Address:
Nand Lal Sharma
Department of Dermatology and Venereology, RPGMC, Kangra (Tanda), Himachal Pradesh
India
| How to cite this article: Jindal N, Sharma NL, Mahajan VK, Shanker V, Tegta GR, Verma GK. Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results. Indian J Dermatol Venereol Leprol 2011;77:148-155 |
Abstract
Background: There is a strong need to develop a photopatch test tray suitable for Indian patients of photodermatitis as European/Scandinavian photopatch test trays may not be wholly relevant for them. Aim: We carried out this study using photoallergens relevant in the Indian context to determine their relevance in patients of photodermatitis. Methods: Thirty patients (M:F, 23:7) between 19 and 76 years of age of photodermatitis and 10 controls were patch- and photopatch tested with 20 common photoallergens. In addition, the patients were also (photo) patch tested with articles of daily use as and when these were suspected to be the cause. Results: Forty-three positive reactions to one or more antigens were seen in 22 (74%) patients. Fourteen positive photopatch tests to seven allergens were observed in 10 (33%) patients, and nine (30%) of them had a definite relevance. The most common contact allergen was fragrance mix (FM) (30%), followed by p-phenylenediamine (20%) and Parthenium hysterophorous (17%). The definite relevance of the patch- and photopatch tests could be correlated in 47% of these patients. Conclusions: FM is the most common contact and photocontact allergen among the various photopatch test antigens. Although differences in technique and evaluation make direct comparison between different centers difficult, still photopatch testing remains an integral part and gold standard for the work-up of the photosensitive patients.Introduction
Photosensitivity is a poorly understood cutaneous reaction to sunlight probably involving the immune system. Photoallergic dermatitis is caused by direct contact of skin with the causative agent and subsequent exposure to light of a certain wavelength. The common photosensitizing agents include chemicals used in sunscreens, antiseptic agents, fragrances and non-steroidal anti-inflammatory agents. A thorough history, especially of evolution of symptoms and of photoaggravation combined with photopatch testing, is an effective approach for the diagnosis of photodermatitis or unclear photoreactions that cannot be associated with genuine photodermatoses. Photopatch testing helps in determining the sensitizing potentials of commonly used agents. Recommendations differ widely across the various photodermatitis research groups, and include variations in test procedures and interpretation, the range of tests substances, the irradiation doses, the precise irradiating wavelengths, the timing of irradiation, the irradiance used and the delay before reading of results. [1],[2],[3] Despite these variations, approximately 4-20% of the patients who undergo photopatch testing have clinically relevant positive results, eventuating in the diagnosis of photoallergic contact dermatitis. [1],[4] There is no Indian standard photopatch test series available, and few studies carried out in India have used European/Scandinavian photo patch test trays, which may not be wholly relevant for Indian patients. Hence, there is a strong need to develop a photopatch test tray suitable for Indian patients of photodermatitis. In this study, we present the preliminary results of photopatch testing using allergens relevant in the Indian context and their relevance in the Indian patients of photodermatitis.
Methods
Thirty consecutive patients (excluding pregnant/lactating females and children aged <18 years) of clinically diagnosed photodermatitis attending the Outpatient Dermatology Department were patch tested between July 2008 and June 2009. Other exclusion criteria included any systemic or congenital disorder and idiopathic photodermatoses known for photosensitivity. Patients having acute dermatitis were enrolled after the acute episode subsided or when the dose of prednisolone was <20 mg/d. Clinical details regarding age, sex, occupation, duration and progress of dermatitis, aggravating factors for photoaggravation and treatment (topical or systemic, particularly photosensitizing drugs) taken were recorded and lesions were charted. Ten subjects were also selected as controls from patients having minor dermatoses other than dermatitis or photodermatitis.
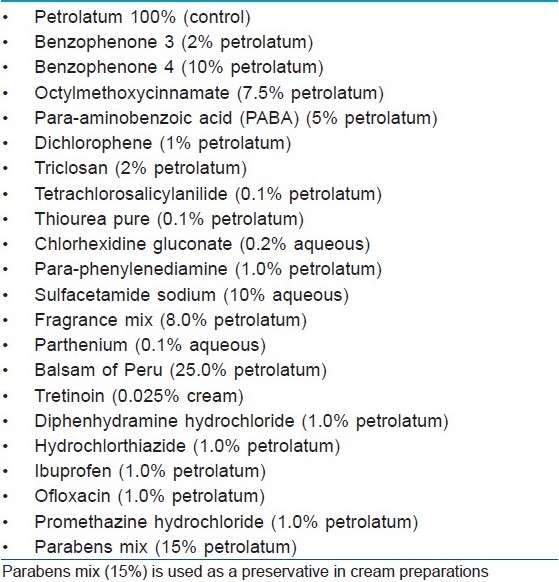
The patch test antigens comprising 20 common photoallergens [Table - 1] were photopatch tested by the Finn Chamber method after obtaining written consent. Para-phenylenediamine (PPD), balsam of Peru (BP), fragrance mix (FM), parthenium and paraben mix were taken from the Indian Standard Series [5] (Systopic India Ltd., New Delhi, India). Sulphacetamide (10% aqueous), chlorhexidine gluconate (0.2% aqueous) and tretinoin (0.025% cream) were tested as such from commercial preparations. The chemicals of other antigens were procured from Himedia Laboratories Pvt. Ltd. Mumbai India and oral drugs were taken from commercially available tablet forms. Antigens were then prepared in the departmental laboratory in standard concentrations with petrolatum as the vehicle. Paraben mix included in this series is used as a preservative in tretinoin cream. All the allergens were stored at 2-8ºC. In addition, the patients were also patch/photopatch tested with articles of daily use as and when these were suspected to have caused photodermatitis.
The patch test units were applied as per the standard procedure in two sets and were kept covered with a radioopaque sheet. After 48 h, one set of patches was removed and exposed to 10 J/cm 2 of UVA (tubes from Philips Holland - TL/10R with dosimeter calibration). After irradiation, the other set of patches was also removed and both the sites were then covered again with the opaque sheet and the patient was asked to come after another 48 h for reading. Readings were taken at 48 and 96 h in all patients and, if needed, at 120 h for reading of the late reaction.
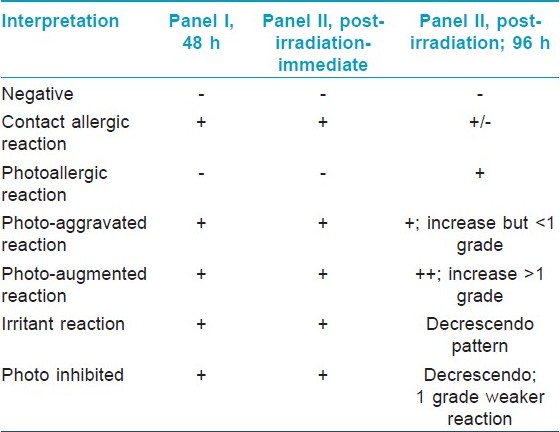
Patch test results were graded according to the International Contact Dermatitis Research Group criteria. [5] Photopatch test results were interpreted according to the criteria shown in [Table - 2]. Photo-aggravated reactions are interpreted as contact allergy with photoaggravation while photo-augmented reactions signify both contact and photocontact allergy.
The relevance of the patch and photopatch test results was defined as "definite" if the reaction is positive to the patch test allergen, object or product containing the suspected allergen; "probable" if the substance identified by the patch test could be verified as present in the known skin contactants; "possible" if the patient is exposed to circumstances in which skin contact with the material known to contain the putative allergen likely occurred; "past" if a positive patch test reaction could be explained by a previous and unrelated episode of contact dermatitis; and "unknown" if there is no evidence of relevance even after extensive investigations.
Results
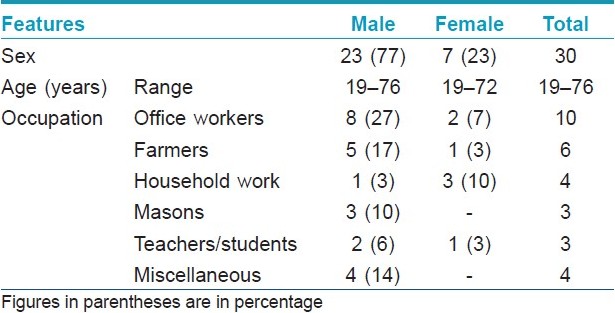
These 30 patients (M:F, 23:7) were between 19 and 76 years of age, and the majority 16 (53%) were in the age group of 41-60 years [Table - 3]. All patients were engaged in various occupations that involved working outdoors some or the other time in the sun, and had a characteristic clinical picture of photodermatitis for the duration varying from 12 days to 30 years with remissions and relapses. Five (16.67%) patients showed generalized body involvement. Twenty-three (77%) patients presented within 5 years while seven (23%) patients had dermatitis for >5 years at the time of presentation. All patients except one had history of exacerbation of dermatitis few minutes to 4 h after sun exposure. The exacerbating factors were: summer season in 15 (50%) patients, insecticides spraying in three (10%) and exposure to parthenium plant in 10 (33.33%). History of drug intake prior to dermatitis was present in five (16.66%) patients (paracetamol, nimesulide, anti-tubercular drugs and tetracycline), although there was no definite history of aggravation of dermatitis with these drugs. Use of hair colorants and cement aggravated dermatitis in two (6.67%) and one (3.33%) patients, respectively.
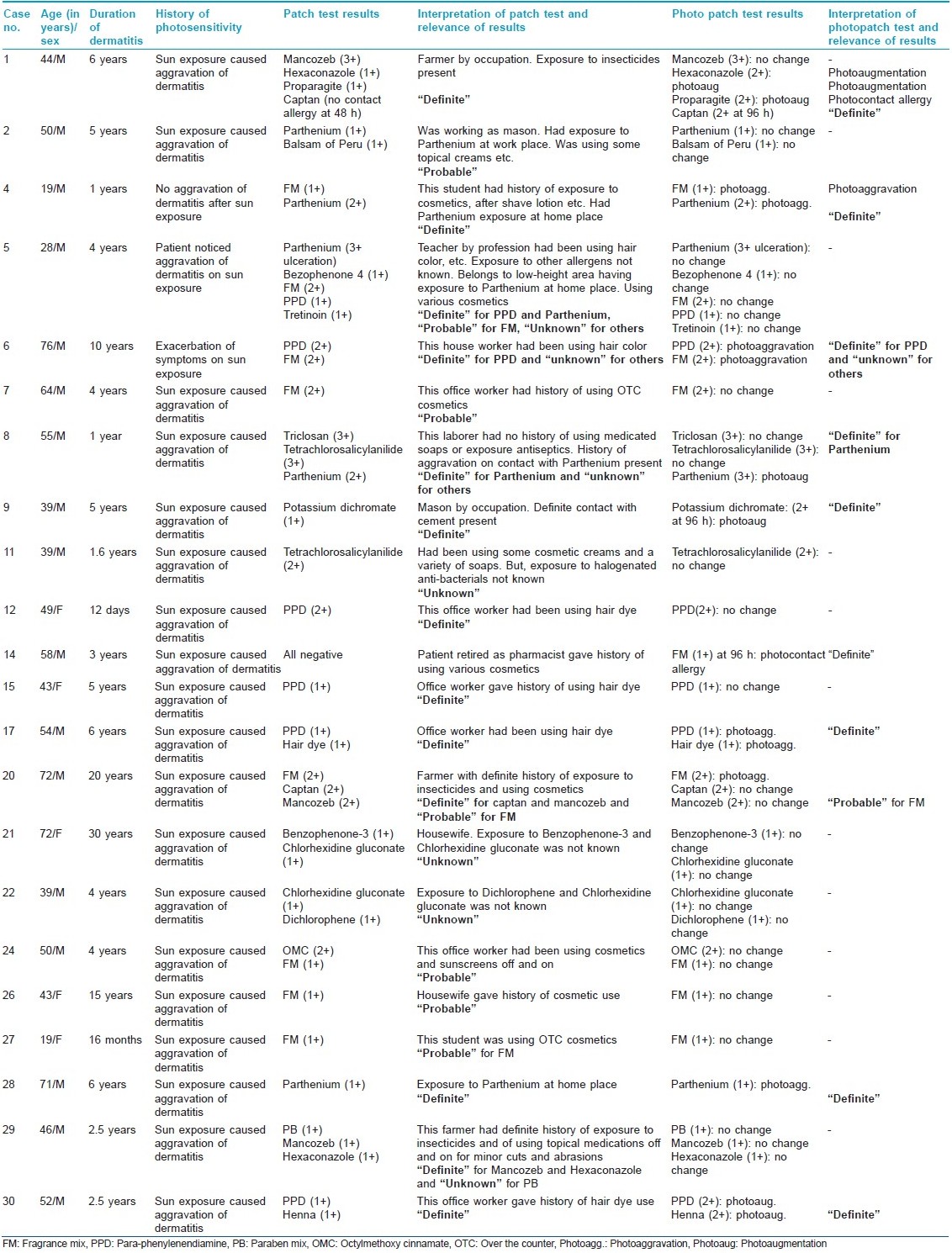
Forty-three positive test reactions were observed to 18 antigens in 22 (73.33%) subjects [Table - 4]. FM was the most common contact allergen (nine patients). Of these nine patients, one showed photocontact allergy after 96 h while three showed photoaggravation. PPD was the next common allergen (six patients), with two patients showing photoaggravation and one exhibiting photoaugmentation. Parthenium hysterophorus showed positive patch test reactions in five (16.67%), with two and one patients exhibiting photo-aggravated and photo-augmented reactions, respectively. Among the pesticides/insecticides group, mancozeb, hexaconazole, captan and proparagite exhibited contact sensitivity in three, two, two and one patients, respectively. One patient exhibited a definite photoallergy to captan while one each showed photo-augmented reactions to hexaconazole and propargite. Positive photopatch tests were definitely relevant in nine (30%) patients and of probable relevance in one. Two controls, both females, were sensitive to tretinoin and FM, but exhibited no photosensitivity.
Discussion
The clinicodemographic profile of our patients of photodermatitis does not differ from what is already reported in the literature. [6],[7],[8],[9],[10],[11] Clinically relevant positive photopatch tests have been observed in 4-20% of the patients in previous studies [1],[4] as compared with 30% in our study. However, this being a small study, the overall prevalence of photoallergens in the population cannot be interpreted.
Exposure to FM occurs commonly through cosmetics and toiletries, food items, other household products (room fresheners, waxes, polishes and insect repellents) and industrial products like metal working fluids. It was the most common contact allergen (30%) in our study, with one (3%) subject having definite photocontact allergy and three (10%) showing a photo-aggravated reaction. Various other studies have also documented positivity, varying from 2 to 21%. [3],[4],[6],[7],[11],[12] Establishing the relevance of FM sensitivity is a difficult task. Most of our patients were using a multitude of products like detergents, soaps and cosmetics, and had a fair chance of developing contact sensitivity to FM. BP is generally included in standard screening patch test series as an indicator of fragrance sensitivity, and shows positive reactions in about 50% of the cases of fragrance allergy when tested with both. [7],[13] Positive photopatch test (phototoxic and photoallergic) results due to BP were ranging from 1.2 to 10.2% in various studies [6],[7],[14] as compared with one (3.33%) patient in our study. However, none of our patients had a concurrent positive patch test to FM and BP.
Six (20%) patients showed contact allergy to PPD, with one of them having photo augmentation and two (7%) having photo aggravation. PPD is a constituent of cosmetic hair colorants, pharmaceuticals and rubber and has been implicated for photocontact sensitivity or persistent light reactions in sensitive individuals. LeVine [15] demonstrated a positive photopatch test to caine mix and PPD and UVA photosensitivity in a 61-year-old man having a recurrent, summer-exacerbated chronic dermatitis. However, PPD is not included for photopatch testing in most standard series. Our patients perhaps developed contact dermatitis/photo contact dermatitis/photoaggravation to PPD from hair colorants.
In India, Parthenium hysterophorus is perhaps the most common cause of contact dermatitis/airborne contact dermatitis, with an element of photosensitivity in some cases. Photo contact dermatitis secondary to P. hysterophorus too has been reported. [16] Understandably, sensitivity to Parthenium is expected to be high due to its profuse and widespread growth and its high sensitizing property. However, its photosensitizing potential remains debatable. While Sharma et al.[17] could correlate P. hysterophorus causing photo contact allergy in four and photoaggravation in six patients, Srinivas et al.[18] did not observe any photosensitivity. In the present study, P. hysterophorus showed a positive patch test reaction in five (16.67%) patients, with one patient having a photo-augmented reaction and two patients showing photo-aggravated reactions. This perhaps signifies that some of these patients of Parthenium sensitivity may show contact allergy with photoaggravation or both, i.e. contact allergy and photo contact allergy. The photo component of Parthenium sensitivity might possibly be due to some allergens of Parthenium hitherto unrecognized or to some other additional allergens unrelated to Parthenium.
Two (6.67%) patients each showed a positive patch test reaction to tetrachlorosalicylanilide (TCSA) and chlorhexidine gluconate, respectively. Although during the 1960s various studies [19],[20] showed TCSA as an important photo contact sensitizer among soap dermatitis patients, Wilkinson [21] observed no reactions in normal persons. Withdrawal of TCSA from soaps subsequently resulted in a decline of the photosensitivity. Similarly, the incidence of contact sensitivity to chlorhexidine is quite low. [6],[7],[11] Contact sensitivity to octylmethoxy cinnamate, benzophenone-3, benzophenone-4, potassium dichromate, dicholorophene, triclosan, paraben mix and tretinoin was observed in one (3.33%) patient each. Among the various topical sunscreens, contact and photo contact allergy to para-aminobenzoic acid and its esters [2],[4],[22] has reduced considerably while benzophenone [9],[23],[24] is being observed as a frequent photoallergen. Only one patient each showed contact sensitivity to benzophenone-3, benzophenone-4 and octylmethoxy cinnamate without photoallergy or photoaggravation in the present study. Potassium dichromate is not a known photo contact sensitizer, [14],[25] although it is a common contact sensitizer. It gave a photo-augmented reaction in one of our patients, which needs to be emphasized. The incidence of photo contact allergy to dichlorophene, triclosan and parabens is infrequent, and so are our observations. Clinically, tretinoin often produces irritant reactions with photoaggravation, but is not a component of the standard photopatch test series. Not many studies on its photosensitizing potential are available. We had only one (3.33%) patient showing contact allergy to it without any photoaggravation. However, large studies are needed to determine its actual contact and photo contact sensitizing properties. [26],[27]
Significant positive patch test reactions to the patient′s own articles/allergens were to mancozeb (three patients), hexaconazole and captan (two patients each) and proparagite (one patient). These were obtained in three farmers having a definite history of aggravation of their dermatitis after exposure to insecticides/pesticides. Photo contact allergy to captan in one patient and photo-augmented reaction to hexaconazole and proparagite in two other patients were significant observations in the present study. Although there is paucity of data on this, positive photopatch test reactions to captan have been observed by Mark et al. [28]
The North American Contact Dermatitis Group recommends inclusion of thiourea rubber accelerators in the standard photopatch test battery. [29] However, none of our patients showed any type of reactivity to thiourea. Anti-histaminic drugs like promethazine, diphenhydramine, chlorpromazine and fentichlor have been reported to be the common photo contact sensitizers, with 2-15.8% positivity. [6],[7],[11],[12] However, Sharma et al.[17] did not observe any positive reaction to these drugs. Some of the systemically administered non-steroidal anti-inflammatory drugs, especially ketoprofen, ibuprofen, diclofenac and piroxicam, were also the common photo contact sensitizers in a multicentric photopatch test study. [30] Similarly, hydrochlorthiazide, a diuretic and commonly used anti-hypertensive, is a well-known photosensitizer, especially in patients sensitive to sulphonamides. [31] Flouroquinolones too can cause phototoxic reactions. [32],[33] In the present study, none of the patients showed any contact or photo contact allergy to any of the systemic drugs patch tested, signifying a dissociation between photosensitivity following ingestion of drugs and contact photosensitivity.
Polysensitivity was observed in 13 (43.33%) patients who showed sensitivity to ≥2 allergens; one patient had sensitivity to a maximum of five patch test allergens. Such multiple positive patch test reactions perhaps occur because of either cross-reactivity, simultaneous exposure to multiple antigens in predisposed individuals or non-specific hyperreactivity. However, none of these allergens showed any photoallergic reactions.
Conclusions
Interpretation and evaluation of relevance of a positive patch-/photopatch test reaction is difficult and intricate. This is evident as we could establish an overall relevance in only 66% of the cases, although relevance in photopatch test-positive patients was high (90%). We feel that insecticides/pesticides need to be studied more comprehensively for their sensitizing and photosensitizing potentials as their exposure, especially inadvertent, is quite frequent in our Indian population. We also feel that a photopatch test tray needs to integrate relevant photosensitizers of past and of today as well as those predicted to be relevant in the future, and need continuous updating.
| 1. |
Neumann NJ, Holzle E, Plewig G, Schwarz T, Panizzon RG, Breit R, et al. Photopatch testing: The 12-year experience of the German, Austrian and Swiss photopatch test group. J Am Acad Dermatol 2000;42:183-92.
[Google Scholar]
|
| 2. |
Thune P, Jansén CT, Wennersten G, Rystedt I, Brodthagen H, McFadden N. The Scandinavian Multicenter Photopatch Test Study 1980-1985: Final report. Photodermatol 1988;5:261-9.
[Google Scholar]
|
| 3. |
Ibbotson SH, Farr PM, Beck MH, Diffey BL, Ferguson J, George SA, et al. British Photodermatology Group Workshop Report photopatch testing-methods and indications. Br J Dermatol 1997;136:371-6.
[Google Scholar]
|
| 4. |
Fotiades J, Soter NA, Lim HW. Results of evaluation of 203 patients for photosensitivity in a 7.3 year period. J Am Acad Dermatol 1995;33:597-602.
[Google Scholar]
|
| 5. |
Sharma VK, Sethuraman G, Garg T, Verma KK, Ramam M. Patch testing with the Indian standard series in New Delhi. Contact Dermatitis 2004;51:319-21.
[Google Scholar]
|
| 6. |
Kanchan PA, Shenoi SD, Balacharan C. Five Year Experience in photopatch test in 50 patients. Indian J Dermatol Venerol Leprol 2002;68:86-7.
[Google Scholar]
|
| 7. |
Panja A, Srinivas CR, Shenoy SD, Balachandran C. Patch photopatch test at Manipal. Indian J Dermatol Venerol Leprol 1994;60:337-9.
[Google Scholar]
|
| 8. |
Bryden AM, Moseley H, Ibbotson SH, Chowdhury MM, Beck MH, Bourke J, et al. Photopatch testing of 1155 patients: Results of the U.K. multicentre photopatch study group. Br J Dermatol 2006;155:737-47.
[Google Scholar]
|
| 9. |
Berne B, Ros AM. 7 years experience of photopatch testing with sunscreen allergens in Sweden. Contact Dermatitis 1998;38:61-4.
[Google Scholar]
|
| 10. |
Pigatto PD, Guzzi G, Schena D, Guarrera M, Foti C, Franacalanci S, et al. Photopatch tests: An Italian multicentre study from 2004 to 2006. Contact Dermatitis 2008;59:103-8.
[Google Scholar]
|
| 11. |
Zeeli T, David M, Trattner A. Photopatch tests: Any news under the sun? Contact Dermatitis 2006;55:305-7.
[Google Scholar]
|
| 12. |
DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis: Results of photopatch testing in New York, 1985- 1990. Arch Dermatol 1992;128:1513-8.
[Google Scholar]
|
| 13. |
Rietschel RL, Fowler JF Jr. Photocontact dermatitis. In: Rietschel RL, Fowler JF Jr, editors. Textbook of Fischer's Contact Dermatitis. 5 th ed. Philadelphia: Lippincott William and Wilkins; 2001. p. 344-50.
th ed. Philadelphia: Lippincott William and Wilkins; 2001. p. 344-50.'>[Google Scholar]
|
| 14. |
Kroon S. Standard photopatch testing with waxtar, para-aminobenzoic acid, potassium dichromate and balsam of Peru. Contact Dermatitis 1983;9:5-9.
[Google Scholar]
|
| 15. |
LeVine MJ. Idiopathic photodermatitis with a positive paraphenylenediamine photopatch test. Arch Dermatol 1984;120:1488-90.
[Google Scholar]
|
| 16. |
Bhutani LK, Rao DS. Photocontact dermatitis caused by Parthenium hysterophorous. Dermatologica 1978;157:206-9.
[Google Scholar]
|
| 17. |
Sharma VK, Sethuraman G, Bansal A. Evaluation of photopatch test series in India. Contact Dermatitis 2007;56:168-9.
[Google Scholar]
|
| 18. |
Srinivas CR, Shenoi DS. Minimal erythema dose to ultra-violet light in parthenium dermatitis. Indian J Dermatol Venereol Leprol 1994;60:149-50.
[Google Scholar]
|
| 19. |
Freeman RG, Knox JM. The action spectrum of photocontact dermatitis caused by halogenated salicylanilide and related compounds. Arch Dermatol 1968;97:130-6.
[Google Scholar]
|
| 20. |
Harber LC, Harris H, Baer RL. Structural features of photoallergy to salicylanilides and related compounds. J Invest Dermatol 1966;46:303-5.
[Google Scholar]
|
| 21. |
Wilkinson DS. Photodermatitis due to tetrachlorosalicylanilide. Br J Dermatol 1961;73:213-9.
[Google Scholar]
|
| 22. |
Szczurko C, Dompmartin A, Michel M, Moreau A, Leroy D. Photocontact allergy to oxybenzone: Ten years of experience. Photodermatol Photoimmunol Photomed 1994;10:144-7.
[Google Scholar]
|
| 23. |
Gonzalez E, Gonzalez S. Drug photosensitivity, idiopathic photodermatosis, and sunscreens. J Am Acad Dermatol 1996;35:871-85.
[Google Scholar]
|
| 24. |
Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens: Review of a 15- year experience and of the literature. Contact Dermatitis 1997;37:221-32.
[Google Scholar]
|
| 25. |
Nethercott J, Paustenbach D, Adams R, Fowler J, Marks J, Morton C, et al. A study of chromium induced allergic contact dermatitis with 54 volunteers: Implications for environmental risk assessment. Occup Environ Med 1994;51:371-80.
[Google Scholar]
|
| 26. |
Fransway AF. The problem of preservation in the 1990s: 3 agents with preservation function independent of formaldehyde release. Am J Contact Dermatis 1991;2:145-74.
[Google Scholar]
|
| 27. |
Marks JG Jr, Belsito DV, DeLeo VA, Fowler JF Jr, Fransway AF, Maibach HI, et al. North American Contact Dermatitis Group patch test results, 1996-1998. Arch Dermatol 2000;136:272-3.
[Google Scholar]
|
| 28. |
Mark KA, Brancaccio RR, Soter NA, Cohen DE. Allergic contact and photoallergic contact dermatitis to plant and pesticide allergens. Arch Dermatol 1999;135:67-70.
[Google Scholar]
|
| 29. |
Rietschel RL, Fowler JF Jr. Photocontact dermatitis. In: Rietschel RL, Fowler JF Jr, editors. Textbook of Fischer's Contact Dermatitis, 5 th ed. Philadelphia: Lippincott William and Wilkins; 2001. p. 408.
th ed. Philadelphia: Lippincott William and Wilkins; 2001. p. 408.'>[Google Scholar]
|
| 30. |
Holzle E, Neumann N, Hausen B, Przybilla B, Schauder S, Hönigsmann HP, et al. Photopatch testing: The 5-year experience of the German, Austrian and Swiss Photopatch Test Group. J Am Acad Dermatol 1991;25:59-68.
[Google Scholar]
|
| 31. |
White IR. Photopatch test in a hydrochlorthiazide drug eruption. Contact Dermatitis 1983;9:237.
[Google Scholar]
|
| 32. |
Hamanaka H, Mizutani H, Shimizu M. Sparfloxacin induced photosensitivity and the occurrence of a lichenoid tissue reaction after prolonged exposure. J Am Acad Dermatol 1998;38:945-9.
[Google Scholar]
|
| 33. |
Scheife RT, Cramer WR, Decker EL. Photosensitizing potential of ofloxacin. Int J Dermatol 1993;32:413-6.
[Google Scholar]
|
Fulltext Views
6,858
PDF downloads
3,514





