Translate this page into:
Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients
2 Apollo Gleneagles Hospitals, Kolkata, India
3 Armed Forces Medical College, Pune, India
4 Assam Medical College and Hospital, Dibrugarh, India
5 Vivekananda Institute of Medical Sciences, Kolkata, India
6 RNT Medical College, Udaipur, India
7 KPC Medical College, Kolkata, India
8 Apollo Hospitals, Chennai, India
9 Bishen Skin Centre, Aligarh, India
10 St. John's Medical College, Bangalore, India
11 Nirvana Skin Clinic, Vadodara, India
12 PSG Hospitals, Coimbatore, India
Correspondence Address:
Abir Saraswat
B 7, Indira Nagar, Lucknow - 226 016
India
| How to cite this article: Saraswat A, Lahiri K, Chatterjee M, Barua S, Coondoo A, Mittal A, Panda S, Rajagopalan M, Sharma R, Abraham A, Verma SB, Srinivas C R. Topical corticosteroid abuse on the face: A prospective, multicenter study of dermatology outpatients. Indian J Dermatol Venereol Leprol 2011;77:160-166 |
Abstract
Background: Abuse of topical corticosteroids (TC), especially over the face, is prevalent worldwide, including in India. Data about the magnitude of this problem in our country is lacking. Aims: The aims of this study were to ascertain the demographics, magnitude and clinical features of TC misuse on the face in the dermatology outpatient department (OPD) attendees in order to raise awareness about this problem and to analyze its causes. Methods: This was a prospective multicenter questionnaire-based clinical study conducted at 12 dermatology centers nationwide. Patients with relevant facial dermatoses reporting to the investigator were asked about their current use of over-the-counter topical formulations and a structured questionnaire applied in case the same was confirmed to be TC. Results: A total of 2926 patients with facial dermatoses were screened, of which 433 (14.8%) were using TC. TC was used as a fairness/general purpose cream or aftershave in 126 (29%) and in 104 (24%) for acne. Steroid combinations were used by 258 (59.6%). Potent and super-potent TC were significantly (P = 0.05) more frequently used by the rural/suburban population. The younger age groups used more potent formulations. A non-physician recommendation for TC use was obtainable in 257 (59.3%) patients. Of these, 232 (90.3%) were for potent/super-potent steroids. Among 176 physician prescriptions, 78 (44.3%) were from non-dermatologists. All non-physician prescriptions and 146 (83%) physician prescriptions for TC were inappropriately refilled. Adverse effects were seen in 392 (90.5%) TC users. Acne/exacerbation of acne was the most common adverse effect. Conclusions: TC misuse in patients with facial dermatoses is quite common, and most of this use is unwarranted. Use as a fairness cream is the most common indication in this cohort. Limitations: This was an OPD-based study and, therefore, it may or may not accurately reflect the community data.Introduction
Topical corticosteroids (TC) are perhaps the most widely used therapeutic agents in modern dermatologic therapy. They provide rapid symptomatic relief in almost all inflammatory dermatoses, especially in the short term. Even incorrect use, for instance in infectious dermatoses, produces an initial improvement in the symptoms. Apart from their anti-inflammatory effect, TC also have potent anti-pruritic, atrophogenic, melanopenic, sex-hormone-like and immunosuppressive effects on the skin. All these can lead to significant local adverse effects if TCs are used indiscriminately. [1] Use of TCs on the face produces peculiar adverse effects in addition to those seen elsewhere, viz. steroid rosacea, acneiform eruption, hypertrichosis, demodicidosis, etc. Another adverse effect seen predominantly on the face has been variously called steroid addiction, [2] dermatitis rosaceaformis steroidica, [3] red face syndrome, [4] etc. by different authors. In this syndrome, after prolonged TC use on the face, there is severe rebound erythema, burning and scaling on the face on any attempted cessation of the application. We have named this condition "topical steroid-dependent face" (TSDF).
In the Indian market, at least 18 different corticosteroid molecules, ranging in potency and activity from mild to super-potent, are available for topical use on the skin. These molecules are marketed under a variety of brand names by thousands of pharmaceutical companies. At least a few of these formulations are available at every medical store with or without a prescription. The situation is further complicated by the inadequate policing of medicine shops by the authorities, whereby any and every medicine, whether over-the-counter (OTC) or not, can be sold without any prescription. To prescribe these agents rationally, India has only a little over 6500 qualified dermatologists to cater to a population of over 1.2 billion. Thus, easy availability of TC and poor access to dermatologists makes the situation in India ripe for their misuse in the community.
TC misuse is well known and has been the subject of studies mainly from Africa [5] and other Asian countries. [6],[7] However, even developed countries like the USA are facing this problem. [8] In spite of the widely perceived enormity of the problem, only a single case series has been published on this problem from India. [9]
This prospective study resulted when a discussion on the online academic forum of IADVL revealed that TC misuse on the face was being seen all over India by many dermatologists, and its incidence appeared to be increasing rapidly. The aim of this study was to ascertain the magnitude, clinical features and demographics of TC misuse on the face in order to raise awareness of this problem in the dermatology community and society at large.
Methods
This was a prospective multicenter questionnaire-type study conducted at 12 dermatology OPDs in different parts of India [Addendum 1 and 2][SUPPORTING:1][SUPPORTING:2]. Patients of any age and of both sexes were recruited consecutively. A questionnaire eliciting demographic variables, characteristics of TC use, prescription source and adverse effects was administered to all eligible patients. Counseling and treatment of TC adverse effects was then started.
Recruitment period
Four months, from April 1, 2008 to July 31, 2008 inclusive.
Inclusion criteria
All patients complaining of facial dermatoses (excluding dermatosis papulosa nigra, melanocytic nevi, adnexal tumors and xanthelasmata) reporting to the investigator were asked the following screening question: "Are you currently using any cream/ointment/lotion on your face that is only available in medical stores?" In the event of a positive answer, the investigator ascertained whether the cream in question contained a corticosteroid by seeing the prescription/used tube or by showing samples of popularly used preparations.
The total number of patients with facial dermatoses seen during the recruitment period was also noted on a separate list (only name, age and sex). Full questionnaires were only filled for those answering "yes" to the screening question.
Current use was defined as any continuous use of seven or more consecutive days or intermittent use over a period of 15 or more days. This use should have been going on till the day of presentation to the center, or if stopped, not more than 15 days before. Investigators were asked to judge whether the TC use in each case was appropriate and justified. Wrong indication (e.g., acne), undiagnosed dermatosis (in investigator′s opinion), inappropriate potency or more than 1 month′s use after the last consultation were criteria used to define unjustifiable/inappropriate use. TSDF was diagnosed in patients who had diffuse erythema over most of the face with or without papules and who complained of subjective local symptoms on stopping the TC application.
Exclusion criteria
Patients not consenting to answering the questionnaire or patients with comorbidities that resembled/could cause changes similar to TC side-effects (e.g., polycystic ovaries/Cushing′s syndrome/thyroid disorders) were excluded from the study.
Statistical analysis
Discrete variables were compared using Student′s t-test and continuous variable were compared using the Chi-squared test. Significance levels were P <0.05.
Results
In all, 2926 patients (1093 male, 1833 female) with facial dermatoses were screened at the 12 centers over the study period. Of these, 433 patients (112 male, 321 female) or 14.8% were found to be using TC on their face. The ratio of females using TC on the face was significantly higher (P < 0.001) than that in the screening population. The mean age of the screening group was 30.1 years (range, 0.5-79 years), which was not significantly different from that of those using TC (mean, 28.3 years; range, 1-73 years).
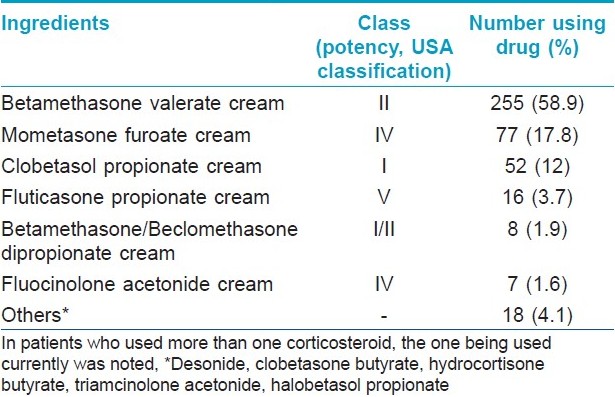
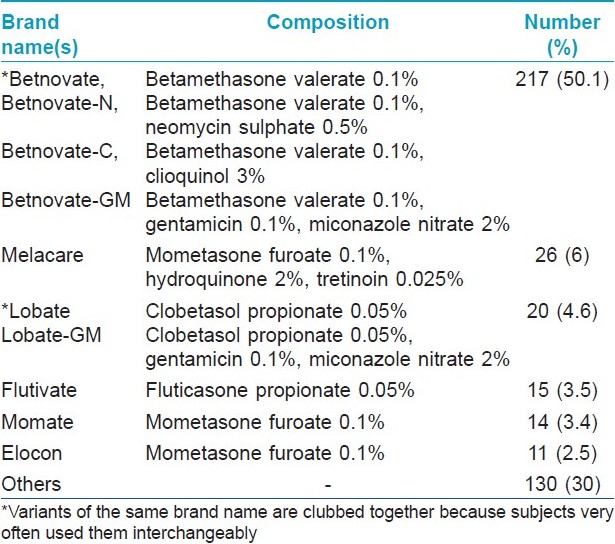
Of the 433 patients in the study group, the largest number (n = 156; 36%) was in the 21-30 years age range. Almost 16% of the study group patients were illiterate and a further 51% had only studied till class 12. Most of the patients (n = 234; 54%) belonged to urban areas, followed by those hailing from suburban areas (n = 118; 27%). A total of 98 different brands containing 12 different TC alone or in various combinations with antifungals, antibacterials or antipruritic agents were identified. Details of the most commonly used TC molecules are shown in [Table - 1]. As regards brand names, Betnovate TM and its variants were by far the largest group, being used by 217 (50.1%) patients. Further details of brands used and their composition are given in [Table - 2]. A total of 258 patients (59.6%) were using combination formulations, while the remaining were using pure TC-containing products. With respect to area of face exposed to TC, 278 patients (63%) were using them all over the face, with the rest using them only over the affected areas.
The pattern of use of TCs was further elucidated and analysis of data revealed that 281 patients (65%) used these products on their face regularly whereas the rest used them intermittently. The duration of use varied widely, ranging from 1 week to 30 years. Details of duration of use are presented in [Table - 3]. The amount of TC used in a month varied from <5 g to 50 g, with 190 patients (44%) using between 11 and 20 g per month.

The source of prescription was ascertained, and we found that 257 patients of 433 (59.3%) had received the recommendation to use TC on the face from a non-physician source. Of these, 129 (50.2%) had been recommended the TC by a friend, peer or relative, 69 (26.8%) directly by the pharmacist, 20 (7.8%) by a beautician and 39 (15.1%) did not remember the source of the recommendation. In the 176 prescriptions from a medical practitioner, 98 (55.7%) were from dermatologists, 47 (26.7%) from MBBS doctors, 20 (11.3%) from other specialists and 11 (6.3%) from practitioners of alternative systems of medicine. According to the criteria used (vide supra), TC application was rated as inappropriate use in all 257 cases in which it was prescribed by a non-physician. In the 176 physician prescriptions, 146 (83%) were rated as inappropriate use.
Underlying dermatoses or problems for which the TCs were used, were general face cream/fairness cream/after shave cream in 126 (29%), for treating acne in 104 (24%), as a lightening agent in melasma in 73 (17%), for other facial hyperpigmentations in 50 (11.5%), others (Tinea, rosacea, facial dermatitides, etc.) in 61 (14%) and undiagnosed rashes in 19 (4.5%). None of the 126 patients who used TCs as fairness/general cream had received this prescription from a physician.
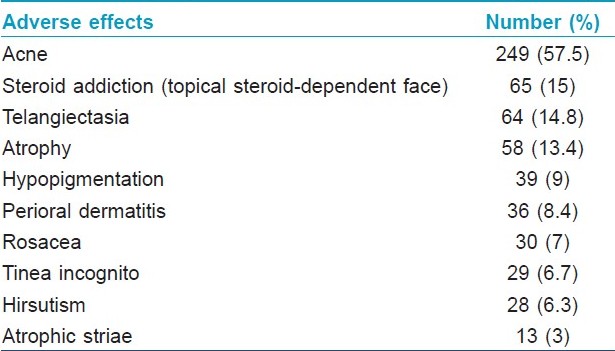
A total of 611 local adverse effects were noted in 392 of the 433 patients (90.5%). More than one adverse effect was seen in 141 (32.6%) patients. Acne, either de novo or an exacerbation of pre-existing lesions, was the most common adverse effect, followed by topical steroid addiction. Atrophic striae on the face were seen 3% of the patients. Further details of adverse effects are presented in [Table - 4].
For further analysis, halobetasol propionate, clobetasol propionate, betamethasone dipropionate, beclomethasone dipropionate and betamethasone valerate were clubbed together in a group called "potent steroids," and all others were clubbed into another group called "milder steroids." When the number of patients using these two groups were compared against their area of residence, it was found that potent steroids were significantly more frequently used in the rural and suburban areas compared with the urban areas (P = 0.052). Patients′ educational status did not seem to play a role in determining use of potent vs. milder steroids (P = 0.42). In the age group of 11-20 years, twice as many patients were using potent steroids compared with those using milder steroids (P = 0.001). In the other age groups, this difference was not significant. The source of the prescription also affected the choice of the TC group. It was seen that 74 of 176 (42%) prescriptions by doctors were for products in the milder steroid group, whereas 232 of 257 (90.3%) recommendations by non-physicians were for potent steroids (P < 0.001).
Discussion
The chief seduction of TC lies in the rapidity of symptomatic relief in almost any dermatosis. This often prompts the busy physician to reverse the natural order of diagnosis followed by treatment. The problem is worsened when a patient is able to easily get an indefinite number of refills of a single prescription from the local chemist, leading to the production of adverse effects and, sometimes, dependence or addiction to TCs. This is a situation faced by dermatologists in many countries, [5],[6],[7],[8] which was described more than 30 years ago as "serious" in a classic paper by Kligman and Frosch. [2] Since that publication, TC use has increased manifold all over the world. In India, the problem is even more complex, wherein anyone can easily get a class I or II TC without the need to get it prescribed by a physician. Moreover, TCs have acquired a reputation as antiacne, antiblemish and fairness creams in the general population, especially in countries with darker-pigmented races. [5]
This large study from 12 dermatology centers in seven different states of India clearly reveals the pan-Indian problem of TC misuse on the face. Almost 15% of the dermatology outpatients with facial dermatoses are already using TCs when they contact a specialist. Alarmingly, in more than 93% of these cases, the TC is either not needed at all, used for much longer than needed, of the wrong potency or is instituted without a diagnosis of the underlying condition. The picture of a typical TC (ab) user on the face that emerges from this data is that of a young female who uses a potent corticosteroid-containing cream recommended by a friend or relative for beauty, fairness or general skin care purpose without any underlying skin ailment for months at a stretch.
Similar studies have been reported from China [6],[10] and Iraq, [7] where TC abuse appears to be very widespread. The Iraqi study reported that 7.9% of the dermatology clinic attendees had misused TCs compared with almost 15% in our study. Most TC abusers in that study were in the 10-19 years age group, whereas in our study, we found that most patients were in the 20-30 years age group. However, our data was limited to facial use, whereas the Iraqi study reported TC abuse anywhere on the body.
In the recent study on facial TC misuse from China, [6] no prevalence data was given, but the proportion of patients applying TCs to the face without any underlying dermatosis in their study (28.5%) was remarkably close to that in our patients (29%). Acne was the most common adverse effect seen in both studies, but our study reported a more than twice higher prevalence in our patients as in the Chinese study. Acne often worsens in hot and humid weather, and our recruitment period fell squarely in the summer/monsoon period. This factor, and the difference in demographic and climatic conditions, probably accounts for the very high prevalence of acne seen in our patients. The full spectrum of TC adverse effects on the face was seen in our study, including even atrophic striae, which is a very uncommonly seen adverse effect on the face. Some representative photographs of the study subjects with various adverse effects are shown in [Figure - 1].
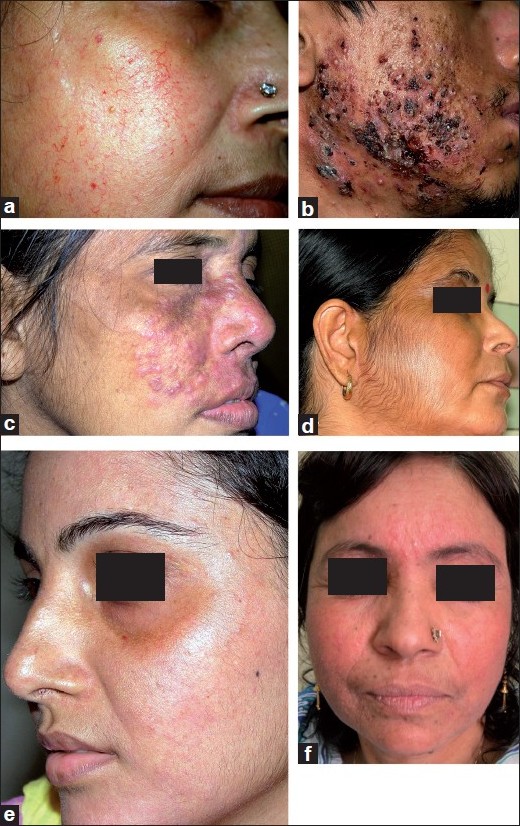 |
| Figure 1: Adverse effects of topical corticosteroids on the face. (a) Marked atrophy and telangiectasia, (b) Severe exacerbation of acne with crusted and nodular lesions, (c) Tinea incognito after prolonged application of a super-potent topical corticosteroid, (d) topical corticosteroid-induced hypertrichosis, (e) hypopigmentation: note sparing of the periorbital area, where the corticosteroid cream was not applied and (f) the "topical steroid-dependent face" with bright erythema and monomorphic papules |
Effective treatment of TC addiction and rosaceiform dermatitis is possible, and results in significant improvement in the quality of life of these patients. [10] Treatment of facial adverse effects of TCs focuses on complete cessation of use, which can be abrupt or gradual, depending on the potency of the product and duration of use. In cases of addiction, progressively less-potent TCs are introduced over a period of weeks to months. Unpleasant symptoms, viz. burning, stinging, pruritus and photosensitivity, are treated using bland emollients, topical calcineurin inhibitors and sunscreens. Systemic agents include tetracyclines, isotretinoin, non-steroidal anti-inflammatory drugs and antihistamines. The subject of pathogenesis and treatment of TC addiction has been reviewed. [11]
In our study, almost 40% of the patients had received TC prescriptions from healthcare providers, whereas this figure was only 28% in the Chinese study. However, this did not translate into increased rate of rational use by the patients, with more than four-fifths of all physician-prescribed TCs deemed inappropriate in our study. In the 98 patients who were using dermatologist-prescribed TCs, either the duration of use was not mentioned in the prescription or it had expired and the patient had continued to use the product.
Most of the subjects were using potent TCs in our study, which is in concordance with prior studies from other countries. [4],[5],[6],[7],[8],[9],[10] Betamethasone valerate alone or in combination was by far the most common corticosteroid used by our patients, and Betnovate TM was the most common brand name. In almost all patients who were using this brand, it had been recommended by a non-physician. Mometasone, hydroquinone and tretinoin-containing skin-lightening formulas have recently become very popular in our country and are being marketed aggressively by numerous pharmaceutical companies to not just dermatologists but all physicians. This has resulted in the oldest brand with this combination (Melacare TM ) becoming the second most commonly misused product in our study.
Patients from rural and suburban areas were found to be much more likely to use potent or super-potent TCs in this study. This is most probably a reflection of poor availability of health care providers in these areas, as our data also shows that non-physicians were much more likely to recommend these products than physicians. Another trend was the high incidence of potent steroid use in teenagers. Preoccupation with appearance and peer pressure probably drive subjects in this age group to use the most potent products available.
This study reveals a part of the problem of TC misuse that is becoming endemic in many countries of the world. Even countries like England, where only hydrocortisone and clobetasone can be sold OTC, are facing the problem of overuse and misuse of these products by the lay public. [12] In India, it appears that the free availability of all TCs without a prescription has allowed many of these brands to become household names, wherein they are no longer considered drugs at all. Patients are unaware of the risks posed by these products and continue to use them for long periods before seeking help from dermatologists. Even correct prescriptions are misused by getting repeated refills from the chemist. At present, loopholes in our laws allow pharmaceuticals to advertise even clobetasol-containing creams on the television and to sell them as OTC products. As indicated by the data in this study, the problem of TC misuse is already significant, and unless urgent steps are taken on all possible fronts, the situation will only get worse and we may soon be facing an avalanche of these unfortunate patients in our clinics.
Recommendations
All healthcare providers need to be sensitized about the dangers of topical corticosteroid misuse, especially on the face.
Legislation/stronger implementation of existing laws is required to limit public access and advertising of potent TC.
Acknowledgment
The authors wish to express their gratitude for the support and assistance to our mentors, Prof. A. K. Bajaj and Prof. V. K. Sharma, and the observer for this study, Prof. S. Sacchidanand.
| 1. |
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54:1-15.
[Google Scholar]
|
| 2. |
Kligman AM, Frosch PJ. Steroid addiction. Int J Dermatol 1979;18:23-31.
[Google Scholar]
|
| 3. |
Basta-Juzbasiæ A, Subiæ JS, Ljubojeviæ S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clin Dermatol 2002;20:135-40.
[Google Scholar]
|
| 4. |
Rapaport MJ, Rapaport V. Eyelid dermatitis to red face syndrome to cure: Clinical experience in 100 cases. J Am Acad Dermatol 1999;41:435-42.
[Google Scholar]
|
| 5. |
Mahe A, Ly F, Aymard G, Dangou JM. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol 2003;148:493-500.
[Google Scholar]
|
| 6. |
Lu H, Xiao T, Lu B, Dong D, Yu D, Wei H, et al. Facial corticosteroid addictive dermatitis in Guiyang city, China. Clin Exp Dermatol 2009;35:618-21.
[Google Scholar]
|
| 7. |
Al-Dhalimi MA, Aljawahiri N. Misuse of topical corticosteroids: A clinical study from an Iraqi hospital. East Mediterr Health J 2006;12:847-52.
[Google Scholar]
|
| 8. |
Solomon BA, Glass AT, Rabbin PE. Tinea incognito and "over- the- counter" potent topical steroids. Cutis 1996;58:295-6.
[Google Scholar]
|
| 9. |
Rathi S. Abuse of topical steroid as cosmetic cream: A social background of steroid dermatitis. Indian J Dermatol 2006;51:154-5.
[Google Scholar]
|
| 10. |
Liu ZH, Du XH. Quality of life in patients with steroid dermatitis before and after treatment. J Eur Acad Dermatol Venereol 2008;22:663-9.
[Google Scholar]
|
| 11. |
Ljubojeviæ S, Basta-Juzbasiæ A, Lipozenèiæ J. Steroid dermatitis resembling rosacea: Etiopathogenesis and treatment. J Eur Acad Dermatol Venereol 2002;16:121-6.
[Google Scholar]
|
| 12. |
Rogers PJ, Wood SM, Garrett EL, Krykant SP, Haddington NJ, Hayhurst J, et al. Use of non-prescription topical steroids: Patients' experiences. Br J Dermatol 2005;152:1193-8.
[Google Scholar]
|
Fulltext Views
10,546
PDF downloads
3,314





