Translate this page into:
Skin tags, leptin, metabolic syndrome and change of the life style
2 Department of Clinical Biochemistry, Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence Address:
Rania M Abdel Hay
13th Abrag Othman, Kournish El Maadi, Cairo - 11431
Egypt
| How to cite this article: El Safoury OS, Abdel Hay RM, Fawzy MM, Kadry D, Amin IM, Abu Zeid OM, Rashed LA. Skin tags, leptin, metabolic syndrome and change of the life style. Indian J Dermatol Venereol Leprol 2011;77:577-581 |
Abstract
Background: Skin tags (STs), are papillomas commonly found in the neck and in the axillae of middle-aged and elderly people. Metabolic syndrome (MS) is a complex of interrelated risk factors for cardiovascular disease and diabetes. Epidemiologic studies of different ethnic populations have indicated that hyperleptinaemia and leptin resistance are strongly associated with MS. Aim: To study the possible relation of skin tags and leptin levels to MS guided by the International Diabetes Federation (IDF) diagnostic criteria. Methods: This study included 80 participants, 40 ST patients and 40 apparently healthy controls. Age, sex, waist circumference (WC), body mass index (BMI), smoking status, fasting glucose level, insulin level and insulin resistance were estimated as well as cholesterol, triglycerides, HDL, criteria of MS, and leptin levels. Results: The univariate analysis showed that WC, BMI, fasting glucose, insulin levels, insulin resistance, cholesterol, triglycerides, HDL, and leptin levels were significantly higher in ST patients compared to controls (P < 0.001). The multivariate analysis between MS components and ST showed that only high triglyceride levels (OR 1.205/95% CI 1.044-1.391/P = 0.011) and low HDL levels (OR 0.554/95% CI 0.384-0.800/P = 0.002) were significantly associated with ST. Multivariate linear regression analysis of the predictors of high plasma leptin levels, showed that high triglyceride levels (OR 0.287/95% CI 0.410-3.56/P = 0.014), and low HDL levels (OR -0.404/95% CI -8.7 to -2.08/P = 0.002) were significant predictors. Conclusion: The results of this study suggested that the presence of both ST and hyperleptinaemia in patients with STs may be associated with high levels of triglycerides and low levels of HDL and this could suggest that changing the life style of patients with ST may have a beneficial role.Introduction
Skin tags (STs), soft fibromas, fibroepithelial polyps, or acrochordons are all alternative terms to describe a common benign skin condition, which consists of a bit of skin projecting from the surrounding skin. [1] Histologically, STs are polypoid lesions with overlying mildly acanthotic epidermis, a loose, edematous fibrovascular core exhibiting mild chronic inflammation. [2] They often develop in areas of skin friction. [3] STs have been reported to be associated with many diseases including type 2 diabetes mellitus (DM) [4],[5],[6] and obesity. [7],[8]
Metabolic syndrome (MS) is a complex of interrelated risk factors for cardiovascular disease (CVD) and DM. These factors include raised blood pressure, dysglycaemia, elevated triglycerides levels, high cholesterol levels, low high-density lipoproteins (HDL), and obesity (particularly central obesity). [9]
Leptin is a 167 amino acid protein that is encoded by the ob gene. [10] Adipocytes are the main site of leptin synthesis and the main contributor to serum leptin levels. Leptin secretion increases with increasing adipocyte cell size, and hence plasma leptin levels are directly proportional to fat mass. [11] Leptin suppresses appetite and increases energy expenditure. [12] It also plays a role in regulation of energy stores. [13] Epidemiologic studies of different ethnic populations have indicated that hyperleptinaemia and leptin resistance are strongly associated with MS and enhance cardiovascular morbidity and mortality through promotion of insulin resistance (IR). [14]
Leptin, as an adipoimmune, may also play a role in the pathogenesis of ST. [15]
Our aim is to study the possible relation of STs and leptin levels to MS guided by the International Diabetes Federation (IDF) diagnostic criteria.
Methods
The present study included 80 participants; 40 patients seeking advice for their STs and 40 age and sex-matched apparently healthy participants serving as controls. This study was conducted in the Dermatology Outpatient Clinic of Cairo University, from August 2009 to December 2010, and was approved by the Dermatology Research Ethics Committee, Faculty of Medicine, Cairo University. All participants were asked to sign an informed consent prior to inclusion in the study. All participants were subjected to thorough history taking including history of smoking, diabetes, hypertension, lipid abnormality, and family history of ST. STs were evaluated by clinical examination, including number, site and color of ST and the presence of acanthosis nigricans. WC was determined and BMI was calculated from the following equation: BMI = weight (kg)/height (m 2 ). Blood pressure was recorded as the average of two measurements after participants had been sitting for 5 min. Diabetes was ascertained according to the WHO criteria or current treatment with antidiabetic agents.
Venous samples were taken at the enrollment visit after the participants had fasted overnight.
All participants had the following laboratory investigations done: Fasting blood glucose using enzymatic colorimetric method (glucose oxidase method), [16] plasma cholesterol and triglycerides by enzymatic colorimetric test, plasma HDL by enzymatic colorimetric method, [17] plasma insulin levels using a commercially available radioimmunoassay (Linco Research, Inc.) and IR was calculated through the Homeostatic Model Assessment (HOMA), [18] plasma leptin level determination by enzyme linked immunosorbent assay (ELISA) using kit supplied by (Linco Research, St. Charles, MO, USA) according to manufacturer instruction.
The MS parameters estimation was recorded according to the guides of the International Diabetes Federation (IDF) consensus worldwide definition. [19]
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (± SD), frequencies (number of cases) and percentages (%) when appropriate. Comparison of quantitative variables between the study groups was done using Student t-test. For comparing categorical data, Chi square (χ2 ) test was performed. Correlation between various variables was done using Pearson′s correlation equation for linear relation. Association between MS and occurrence of STs was tested using stepwise multivariate logistic regression, while the relation to number of STs and plasma leptin levels was done using multivariate linear regression model. P < 0.05 was considered statistically significant. All statistical calculations were done using computer programs Microsoft Excel 2007 (Microsoft Corporation, NY, USA) and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Results
This analytical descriptive study included 40 patients with STs (15 males/25 females) with mean age 41.9 ± 10.47 and mean number of ST lesions 11.1 ± 12.8, 24 patients (60%) presented with flesh colored STs, 9 patients (22.5%) had pigmented STs while 7 patients (17.5%) showed mixed type STs. Another 40 participants, with mean age 41.1 ± 11.56, were enrolled as controls (18 males/22 females). The univariate analysis revealed that patients showed significantly higher WC, higher BMI, fasting glucose levels, and insulin levels as well as higher presence of IR compared to controls (P < 0.001). They also showed significantly higher cholesterol and triglycerides levels, and lower HDL levels (P < 0.001). MS was significantly associated with patients when compared to controls (P < 0.001). Moreover, leptin levels were significantly higher in patients compared to controls (P < 0.001) [Table - 1].
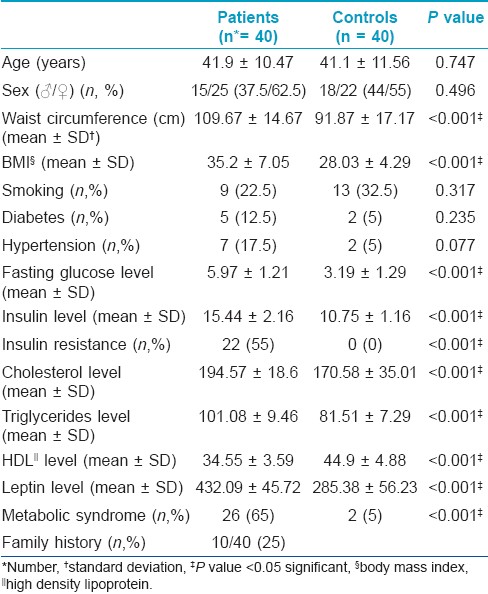
Meanwhile the univariate analysis showed no significant differences between patients and controls as regards age, sex, smoking habits, and the presence of diabetes and hypertension [Table - 1].
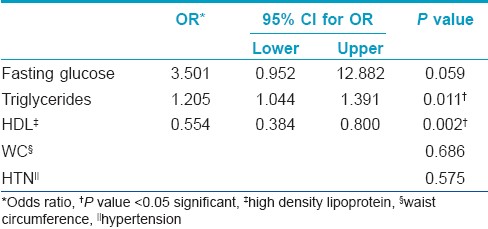
The stepwise multivariate logistic regression of MS components with the occurrence of STs showed that only high levels of triglycerides (OR 1.205/95% CI 1.044-1.391/P = 0.011) and low HDL levels (OR 0.554/95% CI 0.384 0.800/P = 0.002) were statistically significant [Table - 2].
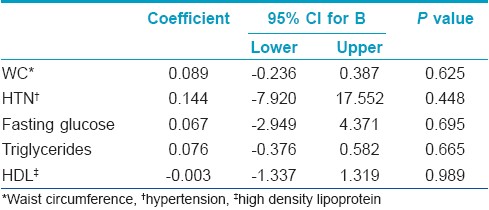
The multivariate linear analysis of MS components with number of STs showed no significant association [Table - 3].
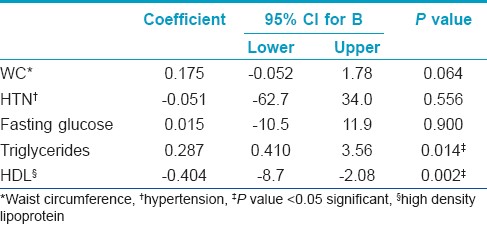
As regards leptin levels, there was no significant correlation between the number of STs in each individual patient and the leptin levels (r = -0.03, P = 0.856). Moreover, the multivariate linear analysis of the predictors of high plasma leptin levels revealed that high leptin levels were significantly associated with high triglycerides levels (OR 0.287/95% CI 0.410-3.56/P = 0.014) and low HDL levels (OR -0.404/95% CI -8.7 to -2.08/P = 0.002) [Table - 4].
Discussion
In this study, STs and/or hyperleptinaemia are related to the presence of dyslipidaemia. We found that although the univariate analysis showed a significant association of MS (65%) with our patients, yet the multivariate analysis revealed significant association of STs with only high triglyceride levels and low HDL levels. Moreover, multivariate linear regression analysis of the predictors of high plasma leptin levels showed that high triglycerides levels and low HDL levels were significant predictors.
To the best of our knowledge, this is the first study to analyze the possible relation of STs and leptin levels to MS.
Our results are in agreement with a study that examined four cases of STs and concluded that STs are a useful sign of increased lipid levels, type 2 DM and CVD. [20]
Our results are also in agreement with a study that investigated cases of STs and proposed that skin tags are cutaneous findings frequently associated with the risk factors for MS and heart disease, and recommended that these patients should be carefully evaluated for MS and heart disease. [21]
Skin tags are common in population and stay long until menopause/andropause. [22] The results of this study advice the screening of patients with ST and hyperleptinaemia for the triglycerides and the HDL (when positive, other components of metabolic syndrome preferably to be measured). This leads us to recommend the change of life style of patients with STs and/or hyperleptinaemia as stopping active smoking and prevention of passive smoking, regular exercises, weight reduction, changing carbohydrate diets into high protein diets. Knowing that diets rich in polyunsaturated fatty acids as olive oil, omega 3, 6, and 9 fatty acids supplementation can decrease the risk of coronary atherosclerosis, [23] we recommend their use for patients with STs and/or hyperleptinaemia.
Acknowledgment
We are indebted to Professor Magdy Ibrahim, MD, Professor of Statistical Unit, Faculty of Medicine, Cairo University, for his statistical analysis of this work.
| 1. |
Millington GW, Graham-Brown RA. Skin and skin disease throughout life. In: Burns A, Breathnach S, Cox N, Griffiths CE, editors. Rook's textbook of dermatology, 8th ed. Oxford: Blackwell Publishing; 2010. p. 8.1-8.30.
[Google Scholar]
|
| 2. |
Gupta S, Aggarwal R, Gupta S, Arora SK. Human papillomavirus and skin tags: Is there any association? Indian J Dermatol Venereol Leprol 2008;74:222-5.
[Google Scholar]
|
| 3. |
Allegue F, Fachal C, Pérez-Pérez L. Friction induced skin tags. Dermatol online J 2008;14:8.
[Google Scholar]
|
| 4. |
Kahana M, Grossman E, Feinstein A, Cohen M, Ronnen M, Millet MS. Skin tags: A cutaneous marker for diabetes mellitus. Acta Derm Venereol 1987;67:175-7.
[Google Scholar]
|
| 5. |
Tompkins RR. Skin tags and diabetes. Arch Dermatol 1977;133:1463.
[Google Scholar]
|
| 6. |
Thappa DM. Skin tags as markers of diabetes mellitus: An epidemiological study in India. J Dermatol 1995;22:729-31.
[Google Scholar]
|
| 7. |
Bhargava P, Mathur SK, Mathur DK, Malpani S, Goel S, Agarwal US, et al. Acrochordon, diabetes and associations. Indian J Dermatol Venereol Leprol 1996;62:226-8.
[Google Scholar]
|
| 8. |
Garcia-Hidalgo L, Orozco-Topete R, Gonzalez-Barranco J, Villa AR, Dalman JJ, Ortiz-Pedroza G. Dermatoses in 156 obese adults. Obes Res 1999;7:299-302.
[Google Scholar]
|
| 9. |
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009;120:1640-5.
[Google Scholar]
|
| 10. |
Buff PR, Dodds AC, Morrison CD, Whitly NC, McFadin EL, Daniel JA, et al. Leptin in horses: tissue localization and relationship between peripheral concentrations of leptin and body condition. J Anim Sci 2002;80:2942-8.
[Google Scholar]
|
| 11. |
Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol 2007;4:1-13.
[Google Scholar]
|
| 12. |
Heshka JT, Jones PJ. A role for dietary fat in leptin receptor, OB-Rb, function. Life Sci 2001;69:987-1003.
[Google Scholar]
|
| 13. |
Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 2006;207:12-22.
[Google Scholar]
|
| 14. |
Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: Linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertension Rep 2008;10:131-7.
[Google Scholar]
|
| 15. |
El Safoury O, Fawzi M, Abdel Hay RM, Hassan AS, El Maadawi Z, Rashed L. Increased tissue leptin hormone level and mast cell count in skin tags: A possible role of adipoimmune in the growth of benign skin growths. Indian J Dermatol Venereol Leprol 2010;76:538-42.
[Google Scholar]
|
| 16. |
Washko ME, Rice EW. Determination of glucose by an improved enzymatic procedure. Clin Chem 1961;7:542-5.
[Google Scholar]
|
| 17. |
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470-5.
[Google Scholar]
|
| 18. |
Wallace T, Levy J, Matthews D. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487-95.
[Google Scholar]
|
| 19. |
Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome: A new worldwide definition. Lancet 2005;366:1059-62.
[Google Scholar]
|
| 20. |
Crook M. Skin tags and the atherogenic lipid profile. J Clin Pathol 2000;53:873-4.
[Google Scholar]
|
| 21. |
Sari R, Akman A, Alpsoy E, Balci MK. The metabolic profile in patients with skin tags. Clin Exp Med 2010;10:193-7.
[Google Scholar]
|
| 22. |
Banik R, Lubach D. Skin tags: Localization and frequencies according to sex and age. Dermatologica 1987;174:180-3.
[Google Scholar]
|
| 23. |
Stone NJ. Focus on lifestyle change and the metabolic syndrome. Endocr Metab Clin North Am 2004;33:493-508.
[Google Scholar]
|
Fulltext Views
5,681
PDF downloads
2,833





