Translate this page into:
Trachyonychia: A comprehensive review
Correspondence Address:
Katherine A Gordon
Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, 1600 N.W. 10th Avenue, RMSB, Room 2023-A, Miami, Florida 33136
USA
| How to cite this article: Gordon KA, Vega JM, Tosti A. Trachyonychia: A comprehensive review. Indian J Dermatol Venereol Leprol 2011;77:640-645 |
Abstract
Trachyonychia or rough nails, may present as an idiopathic disorder of the nails or it can be associated with other dermatological conditions. The dystrophic nail findings seen in trachyonychia are characterized by brittle, thin nails, with excessive longitudinal ridging. The most common histopathologic features associated with trachyonychia are spongiosis and exocytosis of inflammatory cells into the nail epithelia; typical features of lichen planus or psoriasis can also be detected. Determining the cause of trachyonychia is challenging. Treatment is often unsatisfactory, although in general it should be aimed at the underlying cause, if found. In most cases, the nail abnormalities improve spontaneously.Introduction
Trachyonychia, derived from the Greek word trakos, for rough, is a descriptive term referring to rough nail changes. [1] We performed a thorough PubMed search for papers using MeSH terms "trachyonychia, rough nails and twenty nail dystrophy", and compiled this comprehensive review.
The term "twenty nail dystrophy" (TND) is used to describe trachyonychia involving all 20 nails. [2],[3],[4] The nails show diffuse ridging with lack of luster, and in severe cases sandpaper-like surface. [5],[6] In some, the nail plate abnormality may be less severe and one can see numerous, small superficial pits, which impart a shiny appearance to the surface of the nail (shiny trachyonychia). [2]
Trachyonychia was first described by Alkiewicz in 1950 and was termed twenty-nail dystrophy of childhood in 1977 by Hazelrigg, et al.[5],[7] Trachyonychia is much more common in children, with an insidious onset and peak age of 3 to 12 years. [1],[2] However, it can occur at any age. [6],[8] Trachyonychia can be a manifestation of a pleomorphic group of disorders or can be idiopathic. [9] [Table - 1] provides a complete list of reported associations. When associated with alopecia areata, it is more common in male than female patients. [10]
Trachyonychia has been reported to be transmitted in an autosomal dominant fashion in some families [11],[12],[13] and there are reports of monozygotic twins affected by TND. [14],[15] However, these likely represent an association between trachyonychia and alopecia areata, which may occur in twins and in several members of a family.

Idiopathic trachyonychia is likely to be much more common than it is traditionally reported in the literature, as only a few isolated reports of idiopathic trachyonychia have been documented. [2],[16],[17] Determining the cause of trachyonychia when other clinical features are not present can be challenging. Treatment is often unsatisfactory, although in general it should be aimed at the underlying cause, if found. In most cases, the nail abnormalities improve spontaneously.
Clinical Features
The dystrophic nail findings seen in trachyonychia are characterized by brittle, thin nails, with excessive longitudinal ridging [Figure - 1]. This gives the nail plate a rough, opaque appearence. The cuticle is usually hyperkeratotic and ragged. Both the opacity and severity of the nail findings vary in different patients and even in different nails of the same patient. Depending on the severity, it is possibile to distinguish two clinical varieties of trachyonychia: a severe type, characterized by opaque, sandpaper nails, and a mild type characterized by shiny nails with superficial ridging and diffuse pitting. Koilonychia can be seen in both types. [5]
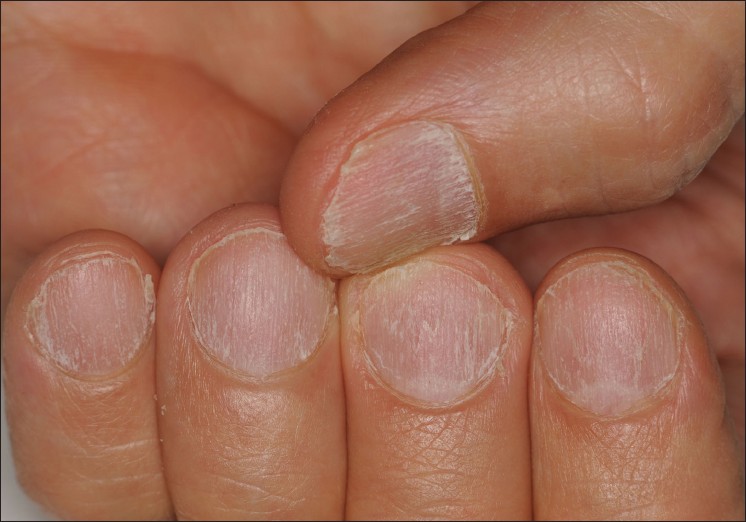 |
| Figure 1: Nail roughness due to excessive longitudinal ridging |
Trachyonychia may present as an idiopathic disorder of the nails with no other cutaneous or systemic findings or it can be caused by a variety of other disorders. [2],[10],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32] It has been reported in association with several diseases such as vitiligo [32] and atopic dermatitis, [31] which are also seen in association with alopecia areata (AA). [10] It is the opinion of the reviewer that these cases most likely represent alopecia areata of the nails. A list of diseases reported in association with trachyonychia can be seen in [Table - 1]; we will focus on reviewing a selected group of them.
Alopecia areata (AA) is the most common disease in patients with trachyonychia, and affects 1-2% of the population worldwide, with a typical onset during childhood or adolescence . Trachyonychia has a prevalence of 3.65% in this patient population . While trachyonychia occurs more frequently in patients with severe alopecia, particularly alopecia universalis, [3] it can also be associated with mild hair loss. Nail changes may precede or follow the onset of AA even by years; most commonly nail changes and hair loss develop simultaneously. Nail biopsies usually demonstrate spongiotic changes; however, there are reports of patients with clinical AA demonstrating the typical features of lichen planus on nail biopsy. [10]
Trachyonychia is seen in 10% of patients affected by nail lichen planus (LP). [25] In these patients, nail LP is most often isolated. However, oral LP is the most common type of LP associated with nail LP. [33] It has been suggested that the form of LP presenting as trachyonychia may be a separate entity as it tends to occur independent of the typical findings of nail LP such as thinning, splitting or nail atrophy. In fact, pterygium formation and scarring is rare in patients with trachyonychia caused by LP, and in general it portends a benign course, sometimes with spontaneous resolution. [17]
Patients with psoriasis and trachyonychia demonstrate similar pathologic changes as in the skin, with acanthosis, focal parakeratosis, and the accumulation of polymorphonuclear cells along the dorsal nail plate. [2] From a morphological point of view, patients who develop trachyonychia in the setting of psoriasis have more thickening of the nail plate as opposed to the thinning seen in lichen planus. Similar to the cutaneous lesions of psoriasis, the Koebner phenomenon can worsen the disease. This has been seen in manual workers such as goldsmiths or jewelry makers, where the digits that are used for daily work are more affected than the others. [34] It is important to note that the diseases chronically involving the nail folds can also lead to trachyonychia.
Diagnosis
In normal nail anatomy and physiology, the nail matrix generates the nail plate, which lies under the proximal nail fold and over the matrix. Trachyonychia is a disease of the nail matrix, thus a pathological diagnosis requires a nail matrix punch or a longitudinal nail biopsy (LNB). However, the pathological diagnosis of trachyonychia is not really required as the disease has a benign outcome, even when caused by lichen planus. We personally do not recommend a nail biopsy in this condition.
The most common histopathologic feature associated with trachyonychia is spongiosis and exocytosis of the inflammatory cells into the nail epithelia. This is seen in trachyonychia associated with alopecia areata and in most cases of idiopathic trachyonychia. [2],[10],[35],[36] Typical pathological findings of psoriasis or lichen planus are seen in trachyonychia caused by these disorders. In rare cases, however, features of nail lichen planus can be observed in the nail biopsy of trachyonychia associated with alopecia areata, indicating that separate diseases can occur simultaneously in the same patient. [10],[37]
Other pathologic changes include nail hypergranulosis, which can be seen as a feature of idiopathic trachyonychia, nail lichen planus, and nail psoriasis. Inflammation due to the disease is traumatic to the nail matrix and can cause a shift in keratinization, where in the keratohyaline granules which are not normally expressed in the nail become abundant. The resultant hypergranulosis is expressed clinically as a physically altered nail plate. This alteration can be permanent even when the inciting inflammation subsides. [38]
Another interesting feature is that commonly the histopathologic changes in all causes of trachyonychia seem to be more prominent in the proximal nail matrix and the ventral proximal nail fold, corresponding clinically to the changes seen in the dorsal nail plate. This suggests that the variations in the nail plate are due to non-uniform levels of inflammatory activity within the nail matrix, seen clinically as sandpaper nails where the level of inflammatory activity is more constant, or as shiny trachyonychia where there are interspersed periods of normal nail matrix function. [2] This hypothesis explains why trachyonychia does not cause permanent scarring, unlike other diseases of the nail matrix.
In conclusion, the risk/benefit ratio of performing a nail biopsy to identify the pathological cause of trachyonychia dictates that a nail matrix biopsy should not be a part of standard procedure.
Differential Diagnosis
The most frequent differential diagnoses to consider are brittle nails, senile nails, alopecia areata, psoriasis, and lichen planus. Brittle nails show longitudinal and superficial splitting but not excessive ridging and roughness. [39] Senile nails show mild longitudinal ridging and beading but these abnormalities are not diffuse involving the entire nail plate. [9] Geometric pitting due to alopecia areata is very similar to shiny trachyonychia. [40]
Lichen planus causes longitudinal fissures and pterygium than that are not seen in trachyonychia. [41]
Treatment
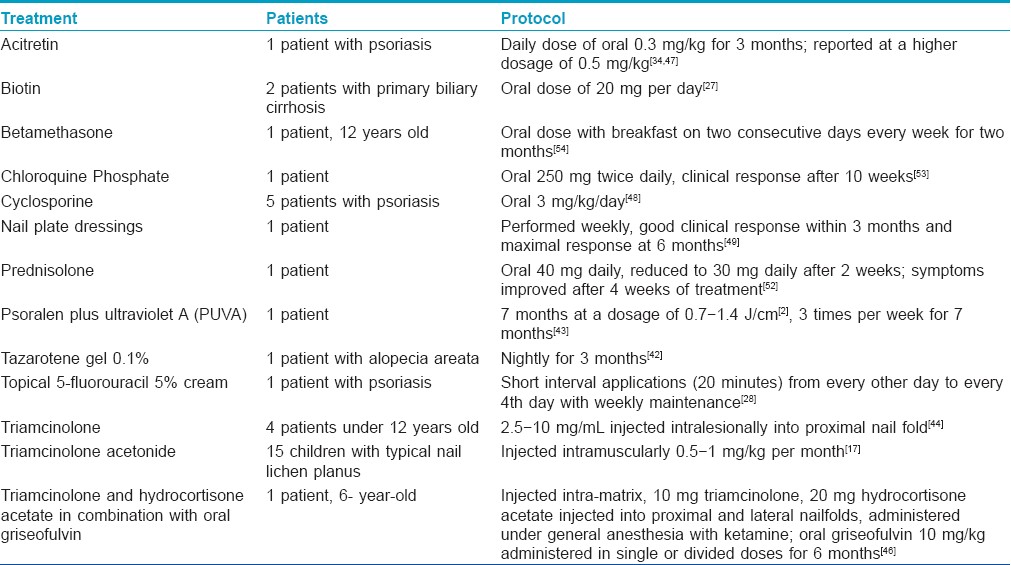
A comprehensive review of the literature reveals that there is no single evidence based treatment for trachyonychia. Therefore, there is no universally accepted treatment for this chronic disorder and many options have been discussed in case reports [Table - 2]. Treatment of trachyonychia is mainly cosmetic as it is not a permanently scarring condition.
In children with TND, a tendency towards spontaneous resolution is seen and one can provide reassurance to parents or to children who are hesitant to have invasive treatments such as corticosteroid injections. [21] One study had determined the rate of complete regression of patients diagnosed with trachyonychia regardless of treatment to be 50%. Of the 12 patients followed up this study, two patients presented with alopecia areata, two others presented with psoriasis, and eight patients did not present with or develop accompanying skin or mucosal disease. [6] Spontaneous resolution may also be seen in patients with trachyonychia associated with alopecia areata. [2]
In patients who desire treatment, there are both topical and systemic options that have been shown in case reports to be successful. [17],[27],[28],[34],[42],[43],[44],[45],[46],[47],[48],[49]
Tazarotene gel 0.1%, a topical retinoid used to treat chronic plaque psoriasis and acne, helped to improve trachyonychia in one patient with alopecia areata after nightly use for three months. [42] Topical chemotherapeutics have also been used with some success in psoriatic trachyonychia. In a case report of one psoriatic patient, topical 5-fluorouracil (5% cream) was used in short interval applications (20 minutes) from every other day to every fourth day with weekly maintenance with clinical improvement. This treatment is limited by periungual irritation. [28]
Psoralen plus ultraviolet A (PUVA) has been effective in one patient with TND who was treated for seven months at a dosage of 0.7?1.4 J/cm 2 three times per week for 7 months. Of note, the untreated toenails remained dystrophic. [43]
Intralesional injection of triamcinolone into the proximal nail fold at a concentration of 2.5-10 mg/ml has been shown to be effective in 4 patients below the age of 12, although relapse is often seen. [44] Patient compliance with treatment continuation is poor secondary to pain. [1],[44],[50],[51] For a 6-year-old boy, Sehgal et al. reported the successful combination of intra-matrix steroids administered under general anesthesia with ketamine and oral griseofulvin (10 mg/kg administered in single or divided doses for six months). [45],[46] The griseofulvin is believed to play an anti-inflammatory role in patients with trachyonychia associated with lichen planus and is best paired with intra-matrix steroids. The ideal model for idiopathic trachyonychia may be intra-matrix steroids used alone. [45],[46]
Tosti et al., [17] assessed the efficacy of intramuscular triamcinolone acetonide 0.5-1 mg/kg per month in 15 children with typical nail lichen planus. Treatment with systemic corticosteroids was effective in curing typical lichen planus, except for the disease recurrence in two children during follow-up. [17]
Systemic retinoids are another treatment option for trachyonychia, and both acitretin and etretinate have been reported as being useful. [47] Tosti et al., [34] reported one case of trachyonychia due to psoriasis, which was treated with oral acitretin at a daily dose of 0.3 mg/kg. [34] After 3 months the nail dystrophy improved, there was less roughness and ridging, and subungual hyperkeratosis and pitting almost disappeared. [34] The use of acitretin for this condition has also been reported at a higher dosage of 0.5 mg/kg. [47] Possible side effects of systemic retinoids include nail-fragility, paronychia, or multiple periungual pyogenic granulomas. [34]
More aggressive therapy in the form of cyclosporine A has been used. In one French study involving patients with psoriasis and trachyonychia, 5 psoriatic patients were treated with cyclosporine at a dose of 3 mg/kg/day. These patients were evaluated using optical profilometry to assess the "roughness" of the nails. Improvement of nail lesions was seen after two to three months of therapy and it lagged behind the cutaneous lesions. [48] Vitamin supplementation of biotin at 20 mg per day has been successful in treating trachyonychia in two patients with primary biliary cirrhosis. [27] Other reported systemic treatments include oral prednisolone (40 mg daily reduced to 30 mg daily after 2 weeks) with symptomatic improvment after 4 weeks of treatment in 1 patient [52] and antimalarials (chloroquine phosphate at 250 mg twice daily) in 1 patient, with recurrence 10 weeks after discontinuation of the antimalarial treatment. [53]
One successful treatment for a child with trachyonychia included systemic corticosteroids. This was achieved with an oral mini-pulse therapy of betamethasone as a single oral dose with breakfast on two consecutive days every week for two months. [54]
Physical modalities have also been used. In one report from Barcelona, nail plate dressings were performed weekly on a 9-year-old patient, who showed good clinical response within 3 months and maximal response at 6 months. [49]
The various treatment modalities for trachyonychia often target the underlying disorder, such as AA, LP, or psoriasis. It is important to note that trachyonychia may be self-limiting in many cases and as such, treatments should only be given when deemed essential. Furthermore, injections into the nail matrix should rarely be considered.
Conclusion
Trachyonychia is a chronic clinical condition that may present as an idiopathic finding or in association with many conditions, especially alopecia areata, psoriasis, and lichen planus. While the histopathological findings have been well documented, the diagnosis of trachyonychia can most often be made based on the distinguishing clinical symptoms. The most effective therapy for trachyonychia has not yet been universally accepted, but in most cases the nail signs improve spontaneously and treatment is not necessary.
| 1. |
Scheinfeld NS. Trachyonychia: A case report and review of manifestations, associations, and treatments. Cutis 2003;71:299-302.
[Google Scholar]
|
| 2. |
Tosti A, Bardazzi F, Piraccini BM, Fanti PA. Idiopathic trachyonychia (twenty-nail dystrophy): A pathological study of 23 patients. Br J Dermatol 1994;131:866-72.
[Google Scholar]
|
| 3. |
Taniguchi S, Kutsuna H, Tani Y, Kawahira K, Hamada T. Twenty-nail dystrophy (trachyonychia) caused by lichen planus in a patient with alopecia universalis and ichthyosis vulgaris. J Am Acad Dermatol 1995;33:903-5.
[Google Scholar]
|
| 4. |
Samman PD. Trachyonychia (rough nails). Br J Dermatol 1979;101:701-5.
[Google Scholar]
|
| 5. |
Baran R, Richert B. Physical signs. In: Baran R, de Berker D, Haneke E, Tosti A, editors. Diseases of the nails and their management. 3 rd ed. London: Blackwell Science; 2001. p. 67-9.
[Google Scholar]
|
| 6. |
Sakata S, Howard A, Tosti A, Sinclair R. Follow up of 12 patients with trachyonychia. Australas J Dermatol 2006;47:166-8.
[Google Scholar]
|
| 7. |
Hazelrigg DE, Duncan WC, Jarratt M. Twenty-nail dystrophy of childhood. Arch Dermatol 1977;113:73-5.
[Google Scholar]
|
| 8. |
Ohta Y, Katsuoka K. A case report of twenty-nail dystrophy. J Dermatol 1997;24:60-2.
[Google Scholar]
|
| 9. |
Singh G, Haneef NS, Uday A. Nail changes and disorders among the elderly. Indian J Dermatol Venereol Leprol 2005;71:386-92.
[Google Scholar]
|
| 10. |
Tosti A, Fanti PA, Morelli R, Bardazzi F. Trachyonychia associated with alopecia areata: A clinical and pathologic study. J Am Acad Dermatol 1991;25:266-70.
[Google Scholar]
|
| 11. |
Pavone L, Li Volti S, Guarneri B, La Rosa M, Sorge G, Incorpora G, Mollica F. Hereditary twenty-nail dystrophy in a Sicilian family. J Med Genet 1982;19:337-40.
[Google Scholar]
|
| 12. |
Balci S, Kanra G, Aypar E, Son YA. Twenty-nail dystrophy in a mother and her 7-year-old daughter associated with balanced translocation 46, XX, t(6q13;10p13). Clin Dysmorphol 2002;11:171-3.
[Google Scholar]
|
| 13. |
Arias AM, Yung CW, Rendler S, Soltani K, Lorincz AL. Familial severe twenty-nail dystrophy. J Am Acad Dermatol 1982;7:349-52.
[Google Scholar]
|
| 14. |
Commens CA. Twenty nail dystrophy in identical twins. Pediatr Dermatol 1988;5:117-9.
[Google Scholar]
|
| 15. |
Karakayali G, Lenk N, Gungor E, Gur G, Alli N. Twenty-nail dystrophy in monozygotic twins. J Eur Acad Dermatol Venereol 1999;12:192-3.
[Google Scholar]
|
| 16. |
Jeanmougin M, Civatte J. Sandy nails and twenty-nail dystrophy of childhood. Apropos of 2 cases. Dermatologica 1984;168:242-6.
[Google Scholar]
|
| 17. |
Tosti A, Piraccini BM, Cambiaghi S, Jorizzo M. Nail lichen planus in children: Clinical features, response to treatment, and long-term follow-up. Arch Dermatol 2001;137:1027-32.
[Google Scholar]
|
| 18. |
Germain-Lee EL, Zinkham WH. Twenty-nail dystrophy associated with hematologic abnormalities. Acta Paediatr Scand 1991;80:977-80.
[Google Scholar]
|
| 19. |
Gupta R, Mehta S, Pandhi D, Singal A. Hereditary Punctate Palmoplantar Keratoderma (PPK) (Brauer-Buschke-Fischer Syndrome). J Dermatol 2004;31:398-402.
[Google Scholar]
|
| 20. |
Palencia SI, Rodriguez-Peralto JL, Castano E, Vanaclocha F, Iglesias L. Lichenoid nail changes as sole external manifestation of graft vs. host disease. Int J Dermatol 2002;41:44-5.
[Google Scholar]
|
| 21. |
James WD, Odom RB, Horn RT. Twenty-nail dystrophy and ichthyosis vulgaris. Arch Dermatol 1981;117:316.
[Google Scholar]
|
| 22. |
Leong AB, Gange RW, O'Connor RD. Twenty-nail dystrophy (trachyonychia) associated with selective IgA deficiency. J Pediatr 1982;100:418-20.
[Google Scholar]
|
| 23. |
Scardamaglia L, Howard A, Sinclair R. Twenty-nail dystrophy in a girl with incontinentia pigmenti. Australas J Dermatol 2003;44:71-3.
[Google Scholar]
|
| 24. |
Crosby DL, Swanson SL, Fleischer AB. Twenty-nail dystrophy of childhood with koilonychia. Clin Pediatr (Phila) 1991;30:117-9.
[Google Scholar]
|
| 25. |
Peluso AM, Tosti A, Piraccini BM, Cameli N. Lichen planus limited to the nails in childhood: Case report and literature review. Pediatr Dermatol 1993;10:36-9.
[Google Scholar]
|
| 26. |
Engineer L, Norton LA, Ahmed AR. Nail involvement in pemphigus vulgaris. J Am Acad Dermatol 2000;43:529-35.
[Google Scholar]
|
| 27. |
Sowden JM, Cartwright PH, Green JR, Leonard JN. Isolated lichen planus of the nails associated with primary biliary cirrhosis. Br J Dermatol 1989;121:659-62.
[Google Scholar]
|
| 28. |
Schissel DJ, Elston DM. Topical 5-fluorouracil treatment for psoriatic trachyonychia. Cutis 1998;62:27-8.
[Google Scholar]
|
| 29. |
Cox NH, Gawkrodger DJ. Nail dystrophy in chronic sarcoidosis. Br J Dermatol 1988;118:697-701.
[Google Scholar]
|
| 30. |
Blanco FP, Scher RK. Trachyonychia: Case report and review of the literature. J Drugs Dermatol 2006;5:469-72.
[Google Scholar]
|
| 31. |
Scher RK, Fischbein R, Ackerman AB. Twenty-nail dystrophy: A variant of lichen planus. Arch Dermatol 1978;114:612-3.
[Google Scholar]
|
| 32. |
Peloro TM, Pride HB. Twenty-nail dystrophy and vitiligo: A rare association. J Am Acad Dermatol 1999;40:488-90.
[Google Scholar]
|
| 33. |
Piraccini BM, Saccani E, Starace M, Balestri R, Tosti A. Nail lichen planus: Response to treatment and long term follow-up. Eur J Dermatol 2010;20:489-96.
[Google Scholar]
|
| 34. |
Tosti A, Bellavista S, Iorizzo M, Vincenzi C. Occupational trachyonychia due to psoriasis: Report of a case successfully treated with oral acitretin. Contact Dermatitis 2006;54:123-4.
[Google Scholar]
|
| 35. |
Jerasutus S, Suvanprakorn P, Kitchawengkul O. Twenty-nail dystrophy: A clinical manifestation of spongiotic inflammation of the nail matrix. Arch Dermatol 1990;126:1068-70.
[Google Scholar]
|
| 36. |
Grover C, Khandpur S, Reddy BS, Chaturvedi KU. Longitudinal nail biopsy: Utility in 20-nail dystrophy. Dermatol Surg 2003;29:1125-9.
[Google Scholar]
|
| 37. |
Kanwar AJ, Ghosh S, Thami GP, Kaur S. Twenty-nail dystrophy due to lichen planus in a patient with alopecia areata. Clin Exp Dermatol 1993;18:293-4.
[Google Scholar]
|
| 38. |
Fanti PA, Tosti A, Cameli N, Varotti C. Nail matrix hypergranulosis. Am J Dermatopathol 1994;16:607-10.
[Google Scholar]
|
| 39. |
Iorizzo M, Pazzaglia M, Piraccini B, Tullo S, Tosti A. Brittle nails. J Cosmet Dermatol 2004;3:138-44.
[Google Scholar]
|
| 40. |
Tosti A, Morelli R, Bardazzi F, Peluso AM. Prevalence of nail abnormalities in children with alopecia areata. Pediatr Dermatol 1994;11:112-5.
[Google Scholar]
|
| 41. |
Tosti A, Ghetti E, Piraccini BM, Fanti PA. Lichen planus of the nails and fingertips. Eur J Dermatol 1998;8:447-8.
[Google Scholar]
|
| 42. |
Soda R, Diluvio L, Bianchi L, Chimenti S. Treatment of trachyonychia with tazarotene. Clin Exp Dermatol 2005;30:301-2.
[Google Scholar]
|
| 43. |
Halkier-Sorensen L, Cramers M, Kragballe K. Twenty-nail dystrophy treated with topical PUVA. Acta Derm Venereol 1990;70:510-1.
[Google Scholar]
|
| 44. |
Khoo BP, Giam YC. A pilot study on the role of intralesional triamcinolone acetonide in the treatment of pitted nails in children. Singapore Med J 2000;41:66-8.
[Google Scholar]
|
| 45. |
Sehgal VN, Sharma S, Khandpur S. Twenty-nail dystrophy originating from lichen planus. Skinmed 2005;4:58-9.
[Google Scholar]
|
| 46. |
Sehgal VN, Abraham GJ, Malik GB. Griseofulvin therapy in lichen planus: A double-blind controlled trial. Br J Dermatol 1972;87:383-5.
[Google Scholar]
|
| 47. |
Brazzelli V, Martinoli S, Prestinari F, Borroni G. An impressive therapeutic result of nail psoriasis to acitretin. J Eur Acad Dermatol Venereol 2004;18:229-30.
[Google Scholar]
|
| 48. |
Pierard GE, Pierard-Franchimont C. Dynamics of psoriatic trachyonychia during low-dose cyclosporin A treatment: A pilot study on onychochronobiology using optical profilometry. Dermatology 1996;192:116-9.
[Google Scholar]
|
| 49. |
Arias-Santiago S, Fernandez-Pugnaire MA, Husein El-Ahmed H, Giron-Prieto MS, Naranjo Sintes R. A 9 year-old child with trachyonychia: a good response with nail plate dressings. An Pediatr (Barc) 2009;71:476-7.
[Google Scholar]
|
| 50. |
Grover C, Bansal S, Nanda S, Reddy BS. Efficacy of triamcinolone acetonide in various acquired nail dystrophies. J Dermatol 2005;32:963-8.
[Google Scholar]
|
| 51. |
Sehgal VN. Twenty nail dystrophy trachyonychia: An overview. J Dermatol 2007;34:361-6.
[Google Scholar]
|
| 52. |
Evans AV, Roest MA, Fletcher CL, Lister R, Hay RJ. Isolated lichen planus of the toe nails treated with oral prednisolone. Clin Exp Dermatol 2001;26:412-4.
[Google Scholar]
|
| 53. |
Mostafa WZ. Lichen planus of the nail: Treatment with antimalarials. J Am Acad Dermatol 1989;20:289-90.
[Google Scholar]
|
| 54. |
Mittal R, Khaitan BK, Sirka CS. Trachyonychia treated with oral mini pulse therapy. Indian J Dermatol Venereol Leprol 2001;67:202-3.
[Google Scholar]
|
Fulltext Views
34,273
PDF downloads
5,117





