Translate this page into:
Physical sunscreens: On the comeback trail
Correspondence Address:
Balaji D More
101 Nirmal Kunj, 299 NL Paralkar Marg, Parel, Mumbai - 400012
India
| How to cite this article: More BD. Physical sunscreens: On the comeback trail. Indian J Dermatol Venereol Leprol 2007;73:80-85 |
Abstract
Awareness of ultraviolet radiation-induced skin damage creates the need for the development of broad-spectrum, safe and cosmetically acceptable sunscreens. Being relatively inert, safe, stable and non-irritating, physical sunscreens are particularly useful for patients with sensitive skin who cannot tolerate chemical sunscreens. However, they form a thick visible pigment layer on the skin. To overcome this drawback, microfine oxides have been developed which made the sunscreens virtually transparent when applied on the skin. This article reviews the rationale for the comeback of physical sunscreens by analyzing data from various sources.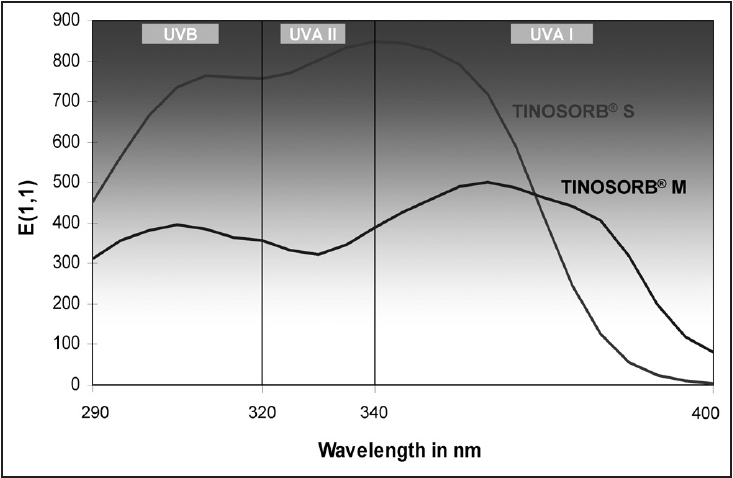 |
| Action spectrum of MBBT Tinosorb� covering UVB, UVA II and UVA I |
 |
| Action spectrum of MBBT Tinosorb� covering UVB, UVA II and UVA I |
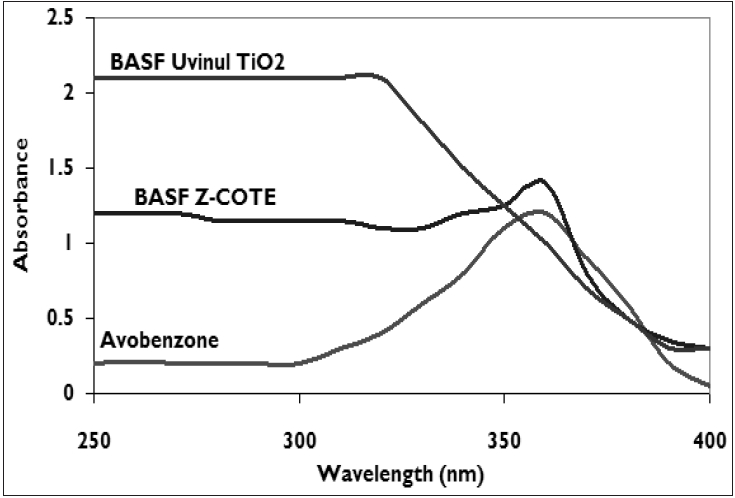 |
| FDA-approved UVA screen absorbance spectra: TiO2- 21 nm primary particle size with 100 nm aggregates, 5% dispersion in Vaseline; Avobenzone-1% in ethanol; Z-Cote-5% dispersion in Vaseline[11] |
 |
| FDA-approved UVA screen absorbance spectra: TiO2- 21 nm primary particle size with 100 nm aggregates, 5% dispersion in Vaseline; Avobenzone-1% in ethanol; Z-Cote-5% dispersion in Vaseline[11] |
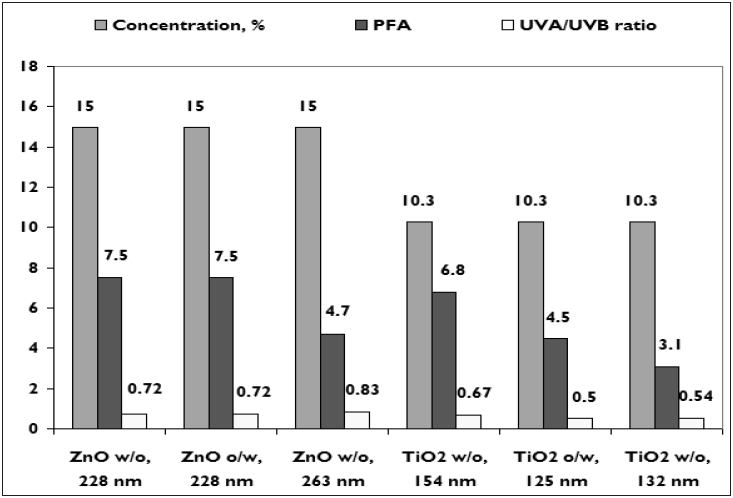 |
| UVA protection factors based on PFA and UVA/UVB ratios for zinc oxide and titanium dioxide for w/o and o/w emulsions[11] |
 |
| UVA protection factors based on PFA and UVA/UVB ratios for zinc oxide and titanium dioxide for w/o and o/w emulsions[11] |
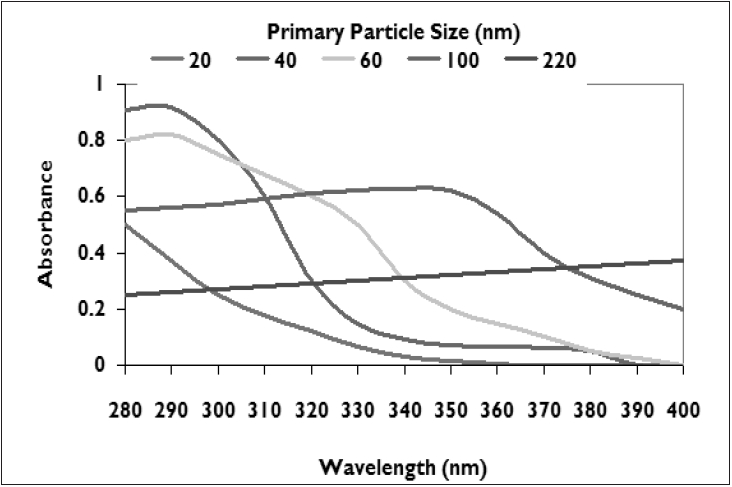 |
| UV absorbance characteristics of titanium dioxide depend on the primary particle size[11] |
 |
| UV absorbance characteristics of titanium dioxide depend on the primary particle size[11] |
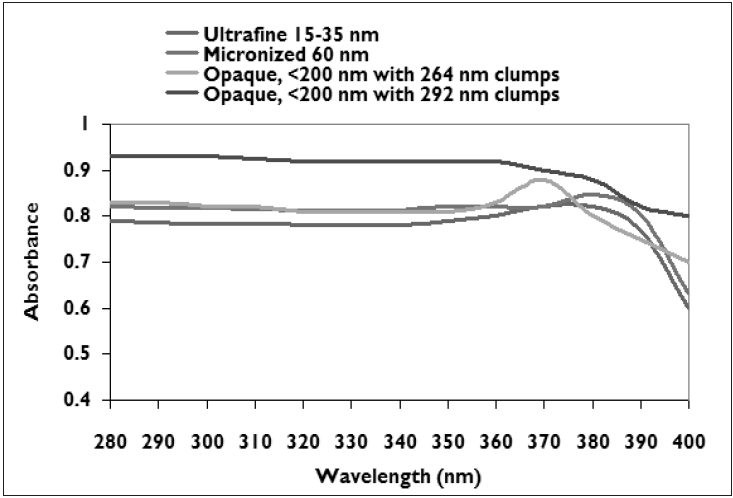 |
| Absorbance spectra for zinc oxide particles of varying sizes[10] |
 |
| Absorbance spectra for zinc oxide particles of varying sizes[10] |
INTRODUCTION
The component of the solar spectrum covering 200-400 nanometers (nm) is responsible for skin damage and is termed ultraviolet radiation (UVR). UVR consists of UVR-B (290-320 nm) and UVR-A (320-400 nm). Substances which physically attenuate UVR by causing molecular rearrangement (size, shape and appearance change) without any effect on the internal structures) are called physical UVR attenuators. Zinc oxide (ZnO), titanium dioxide (TiO 2 ), talc, kaolin, iron oxides, red petrolatum, silica and mica are examples of physical attenuators of UVR of which ZnO and TiO 2 are approved as active sunscreen ingredients. [1]
The only drawback of these agents is that they form a thick visible pigment layer on the skin, which is not acceptable to most individuals. To overcome this drawback, microfine oxides have been developed which have made physical sunscreens virtually transparent on skin. However, there is no universal consensus on the definition of the term ′microfine′. For all practical purposes, the term "microfine" is used to describe particles of sizes ranging from a few nm to several microns whereas pigmentary grade particles fall in the 80 nm to 250 nm size range. [1] However, particle size distribution rather than particle size regulates the efficacy and the transparency in a dispersion.
For hundreds of years, ZnO has been used topically to treat many skin disorders with fairly encouraging results. Zinc is an essential mineral, a component of about 70 metalloenzymes and required for DNA, RNA and protein synthesis. [2],[3] In addition, due to its anti-microbial action, ZnO has been used for dressing burns and other wounds in the pre-antibiotic era. [2] TiO 2 has a variety of uses as it is odorless and absorbent. This mineral can be found in many products, ranging from paint to food to cosmetics. It is usually coated with silica or stearate to reduce photoreactivity. Micronized TiO 2 has been shown to provide protection against UVB-induced immunosuppression in humans in vivo . [4]
Mechanism of UVR Attenuation
It was believed that in contrast to chemical sunscreens, the physical metal oxides act as only scatterers or reflectors of UVR. However micronized forms of metal oxides (ZnO and TiO 2 ) of sizes below 370 nm, owe their UVR attenuating ability to their absorbing power, practically no light being reflected. [5],[6],[7] Whenever light falls on a substance, a certain fraction of the incident light is either reflected, scattered or absorbed. Absorption is a process, in which light is lost, i.e., it is converted into some other form of energy.
Absorption of UVR by metal oxides is evident from the mobilization of electrons within their atomic structure. The energy required for mobilization is absorbed from UVR thus causing these metal oxides to act as photosemiconductors. [1],[8] The energy gap is the amount of energy required to promote the electron from the valence band into the conductive band (3.23 electron Volts (eV) and 3.06 eV for ZnO and TiO 2 respectively). These energy gaps are calculated by using the quantum theory that corresponds to a UV wavelength of 385 nm for ZnO and 405 nm for TiO 2 . [1],[8] Therefore all UV radiations shorter than 385 and 405 nm will be absorbed by ZnO and TiO 2 respectively. The absorbed energy is then emitted as longer wavelength radiations as the electrons return to a lower energy level. This whole event occurs within less than 10.6 seconds. [1] Although metallic oxides are not inert per se, they can be coated to make them stable, non-toxic and safe. [9]
UV Absorption Spectrum
The UVA coverage ability of microfine physical block particles depends primarily on the particle size. [10] Micronized ZnO particles efficiently absorb radiation from the entire UV region (380 nm and shorter) with a steep drop-off whereas TiO 2 particles smaller than 100 nm result in coverage up to 340-360 nm as seen in [Figure - 1][Figure - 2]. [7],[10],[11] About 90% of UVR of wavelength below 360 nm is attenuated by absorption. [1],[8],[11] UVA attenuation is better than UVB attenuation with particles of larger size. [12] Micronized forms have the ability to attenuate both UVB and UVA II with a steep or gradual drop off in absorbance in the upper UVA I range. Attenuation characteristics vary greatly for TiO 2 as seen from the UVB, and UVA absorbance (320-400 nm) spectra. Formulations of ZnO tend to attenuate both UVA and UVB more uniformly than those of TiO 2 . It is prudent to consult the supplier for product absorbance and attenuation curves so as to determine the extent of UVA coverage of a given physical sunscreen.
For ZnO and TiO 2 , particle size of ~0.1 micron is the most effective in attenuating UV radiation. [13] UV blocking capacity decreases as the particle size goes below 0.1 micron whereas above this size, whitening can occur. Very fine particles have a tendency to agglomerate to form large particles. In many cases, larger aggregates are formed during the manufacturing of the powder even before the formulation step. These large aggregates / agglomerates may not be effective depending on the ultimate size. Physical sunscreens can also be formulated by incorporating both ZnO and TiO 2 or their combination with chemical sunscreen ingredients to achieve higher levels of protection. Currently, sun protection factor (SPF) is the only globally accepted scale to estimate a sunscreen′s protective action. However, it does not provide the complete protective profile of a sunscreen especially against long wavelength UVA I (340-400 nm) as it only takes into account UVB radiation.
ZnO and TiO 2 are among three sunscreen ingredients approved by US-FDA to claim adequate coverage in the long-wave range of the UVA spectrum, the third being avobenzone. Evaluating the efficacy of UVA protection will enable true broad-spectrum UVB and UVA photoprotection. [14] However, there is no consensus on the method of evaluating UVA protection. UVA protection factors based on protective factor ability (PFA) and UVA/UVB ratios for zinc oxide and titanium dioxide for (water in oil) w/o and (oil in water) o/w emulsions are seen in [Figure - 3]. [11]
OPTICAL PROPERTIES AND TRANSPARENCY
Microfine metal oxides possess the unique property of being transparent to visible light but opaque to UV radiations. [13] Transparency is explained by the ability to allow visible light to pass through while opacity results from the ability to attenuate UVR transmission by reflection, scattering and /or absorption. The opacity of a suspension of fine material is influenced by the particle size of the material, the difference in the refractive indices of dispersed material and the dispersing medium and the degree of wavelength of light used in the opacity measurement. [13] Refractive index (RI) measures the speed of light in the given substance relative to speed of light in air. [14] Light scatter within any medium is a function of difference in the RIs between the pigment and the medium. If the ratio of the RIs is close to 1, the whole system has a transparent appearance. In contrast, the entire system has a white appearance if the ratio is significantly greater than 1. The RI of ZnO is 1.9 whereas that of TiO 2 is 2.6 making it whiter in appearance. [1]
The RI of a material is intrinsic to that material and is a fixed characteristic. However, it can be minimized by one of two ways: either by using a suspending medium with a RI similar to that of the material or by manipulating the particle size of the material. The first method is limited due to lack of availability and appearance of such suspending media. Hence, the only remaining option, that of reduction of particle size is exploited to reduce the opacity of metal oxides. Therefore, particles less than 0.25 microns in size actually transmit more visible light than their larger counterparts. [13] However, when the particle size is further reduced, the particles tend to agglomerate and aggregate leading to increased "particle" size and opacity. Hence, particle size needs to be maintained in a well-defined range and must be evenly distributed.
PHARMACOKINETICS
ZnO is not absorbed through the skin. Skin absorption studies after zinc oxide application to intact and psoriatic skin revealed essentially unchanged serum zinc levels. [15] In addition, application of 40% zinc ointment to relatively large areas of skin does not raise the serum zinc levels. [2] Although microfine TiO 2 deposited on the outermost surface of the stratum corneum generally does not penetrate the different layers of the skin, [16] one study has demonstrated that it does penetrate the skin in very minute quantities. [17] Therefore, more studies are necessary to establish if dermal absorption of physical sunscreens is statistically and clinically significant.
Although concerns have been raised about a possible photocatalytic activity of metal oxides on living tissues, crystal surface photocatalytic activity has not been encountered with ZnO. [6],[18] The absorption curves for microfine ZnO before and after solar simulator irradiation (30 Lcm 2 ) was similar, demonstrating it to be completely photostable. [7] To reduce potential adverse effects, metal oxides used in cosmetic preparations are often coated. [16] Manganese-doped titania oxides show improved efficacy over undoped TiO 2 in sunscreen formulations containing organic UV absorbers. [19]
CLINICAL USES
Metal oxides as a single ingredient sunscreen can be safely used for daily skin protection from UVR. [20] The metal oxides sunscreen formulations are usually less oily and may prove useful in patients with subjects with oily and acne-prone skin. They offer photoprotection to individuals with visible light and UV-A photosensitivity such as those with porphyria, drug photoallergy and polymorphous light eruption. [21],[22],[23] They are especially useful for patients with sensitive skin and those not tolerating chemical sunscreens.
DIRECTIONS FOR USE
Most "directions for use" on sunscreen containers and packaging recommend that users "apply liberally" or "generously". However such directions are vague and the manufacturer must give the exact method of application. The exact amount of sunscreen required to be applied on the skin for optimal protection should be recommended by the manufacturer based on the method and quantity of the sunscreen formulation used for determination of the SPF. When using physical sunscreens, it is advisable not to rub them too hard as they work best on the surface of the skin. They leave the surface of the skin with a milky glaze which can be quite useful for gauging which skin areas you are covering and which one may have missed. Sweat will remove sunscreen more effectively than water. The ideal sunscreen works by leaving a uniform film on the surface of the skin. Sunscreen should be reapplied whenever it may have been rubbed off. The substantivity of sunscreens will be determined by its water resistance, higher water resistance will confer more substantivity to sunscreens.
SAFETY PROFILE
ZnO is approved as a category I protectant by the US-FDA. [24] Moreover it is approved for use in the treatment of diaper rash because it is safe to apply to inflamed non-intact skin. [25],[26] Titanium dioxide is regarded as an inert, non-toxic substance by many regulatory bodies as seen in many MSDS (Material Safety Data Sheets). It is evident that physical sunscreens absorb UVR and in aqueous environments, can lead to the generation of hydroxyl radicals. [6] This can initiate oxidations and studies with TiO 2 have shown that it produces DNA damage both in vitro and in human cells, indicating that further studies are required to assess the safety of micronized TiO 2 and by extension, of ZnO. However, by providing UV-A protection and by preventing short-term UVB-induced immunomodulation, physical sunscreens are stated to be protective against DNA damage as indicated by two studies. [4],[27]
However, much debate has arisen regarding the safety of nanoparticle use in sunscreens. The Government of Australia, Department of Health and Aging, has reviewed the literature on the safety of nano-particles of ZnO and TiO 2 in sunscreens. It was observed through isolated cell experiments that ZnO and TiO 2 can induce free radical formation in the presence of light and that this may damage these cells (photomutagenicity with ZnO). However, this would only be of concern in people using sunscreens if ZnO and TiO 2 penetrated into viable skin cells. The weight of current evidence is that they remain on the surface of the skin and in the stratum corneum of the skin. [28],[29]
INTERACTION WITH STEROIDS
Prednisolone and zinc react in a molar proportion of 2:1 to form a defined compound. Hence the application of prednisolone with zinc oxide-containing dermatological formulations is not indicated. [30] A fixed-dose topical formulation of ZnO and hydrocortisone had poor stability. Hydrocortisone decomposed mainly to its 21-aldehyde, but other degradation products were also identified. [31]
Organic sunscreens with microfine particle size: A ′physical′ chemical sunscreen (33),(34)
A development among chemical sunscreens is the availability of sunscreen agents with microfine particle size which renders them the property of scattering and reflecting UV rays in addition to absorbing them.
MBBT (Methylene Bis-Bezotriazolyl Tetramethylbutylphenol) (Tinosorb ® ) is the first agent in this new class with an average particle size of 150 nm. It attenuates UV rays by triple action: 85% UV rays are absorbed, 10-15% UV rays are scattered and 3-5% are reflected. It has a broad UV spectral coverage including UVA I with a critical wavelength of 388 nm, PFA of 7.07 and UVA/UVB ratio of 1. It is thus an effective broad spectrum sunscreen.
CONCLUSION
There is overwhelming evidence to support the hazards of UVR. Public awareness of these hazards has increased significantly. Resurgence of physical sunscreens has occurred at the most appropriate time and improved technology has made it possible to make them invisible on skin. Moreover, physical sunscreens show potential to overcome some of the limitations of chemical sunscreens. They are especially useful in patients sensitive to ingredients of chemical sunscreens, in children and for long-term use because of their high safety profile.
ACKNOWLEDGEMENT
I gratefully acknowledge Dr. Carol Demas for giving permission to use the [Figure - 1],[Figure - 2],[Figure - 3],[Figure - 4] depicted in this review article. I acknowledge Dr. Uli Oster Walder and Mr. Uday Kulkarni of Ciba Speciality Chemicals Ltd. for [Figure - 5] depicted in this article.
| 1. |
Fairhurst D, Miltchnick MA. Particulate sun blocks: General principles. In: Lowe, NJ, Shaath NA, Pathak MA, editors. Sunscreens: Development, Evaluation and Regulatory Aspects, 2 nd ed. Marcel Dekker: New York; 1997. p. 313-52.
[Google Scholar]
|
| 2. |
Derry JE, McLean WM, Freeman JB. A study of percutaneous absorption from topically applied zinc oxide ointment. J Parenter Enteral Nutr 1983;7:131-5.
[Google Scholar]
|
| 3. |
Prasad AS. Clinical and biochemical manifestations of zinc deficiency in human subjects. J Am Coll Nutr 1985;4:65-72.
[Google Scholar]
|
| 4. |
van der Molen RG, Hurks HM, Out-Luiting C, Spies F, van't Noordende JM, Koerten HK, et al . Efficacy of micronized titanium dioxide-containing compounds in protection against UVB-induced immunosuppression in humans in vivo . J Photochem Photobiol B 1998;44:143-50.
et al . Efficacy of micronized titanium dioxide-containing compounds in protection against UVB-induced immunosuppression in humans in vivo . J Photochem Photobiol B 1998;44:143-50. '>[Google Scholar]
|
| 5. |
Sayre RM, Kollias N, Robert RL, Bager A, Sadig I. Physical sunscreens. J Soc Cosm Chem 1990;41:103-9.
[Google Scholar]
|
| 6. |
Dunford R, Salinaro A, Cai L, Serpone N, Horikoshi S, Hidaka H, et al . Chemical oxidation and DNA damage catalyzed by inorganic sunscreen ingredients. FEBS Lett 1997;418:87-90.
[Google Scholar]
|
| 7. |
Mitchnick MA, Fairhurst D, Pinnell SR. Microfine zinc oxide (Z-cote) as a photostable UVA/UVB sunblock agent. J Am Acad Dermatol 1999;40:85-90.
[Google Scholar]
|
| 8. |
Anderson MW. Broad-spectrum physical sunscreens: Titanium dioxide and zinc oxide. In: Lowe, NJ, Shaath NA, Pathak MA, editors. Sunscreens: Development, evaluation and regulatory aspects, 2 nd ed. Marcel Dekker: New York; 1997. p. 353-98.
[Google Scholar]
|
| 9. |
Wolf R, Matz H, Orion E, Lipozencic J. Sunscreens-the ultimate cosmetic. Acta Dermatovenerol Croat 2003;11:158-62.
[Google Scholar]
|
| 10. |
Shao Y, Schlossman K. Kobo Products Inc. Effect of particle size on performance of physical sunscreen formulas. PCIA Conference: Shanghai, China; 1999.
[Google Scholar]
|
| 11. |
Demas C, Kwiatkowska B. Biochemistry of beauty: Sunscreens. Available from: http://www.biochemistryofbeauty.com. [Last accessed on 2005 Jan 7].
[Google Scholar]
|
| 12. |
Murphy GM. Sunblocks: Mechanisms of action. Photodermatol Photoimmunol Photomed 1999;15:34-6.
[Google Scholar]
|
| 13. |
Mitchnick MA. Zinc oxide, an old friend to rescue. Cosmetics Toiletries 1992;107:111-6.
[Google Scholar]
|
| 14. |
Grady LD. Zinc oxide in face powders. J Soc Cosm Chem 1947;1:17-21.
[Google Scholar]
|
| 15. |
Gasparro FP, Mitchnick M, Nash JF. A review of sunscreen safety and efficacy. Photochem Photobiol 1998;68:243-56.
[Google Scholar]
|
| 16. |
Lademann J, Weigmann H, Schafer H, Muller G, Sterry W. Investigation of the stability of coated titanium microparticle used in sunscreens. Skin Pharmacol Appl Skin Physiol 2000;13:258-64.
[Google Scholar]
|
| 17. |
Tan MH, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas J Dermatol 1996;37:185-7.
[Google Scholar]
|
| 18. |
Edlich RF, Winters KL, Lim HW, Cox MJ, Becker DG, Horowitz JH, et al . Photoprotection by sunscreens with topical antioxidants and systemic antioxidants to reduce sun exposure. J Long Term Eff Med Implants 2004;14:317-40.
[Google Scholar]
|
| 19. |
Wakefield G, Lipscomb S, Holland E, Knowland J. The effects of manganese doping on UVA absorption and free radical generation of microfine titanium dioxide and its consequences for the photostability of UVA absorbing organic sunscreen components. Photochem Photobiol Sci 2004;3:648-52.
[Google Scholar]
|
| 20. |
Rouabhia M, Mitchell DL, Rhainds M, Claveau J, Drouin R. A physical sunscreen protects engineered human skin against artificial solar ultraviolet radiation-induced tissue and DNA damage. Photochem Photobiol Sci 2002;1:471-7.
[Google Scholar]
|
| 21. |
Schwarz VA, Klein SD, Hornung R, Knochenmuss R, Wyss P, Fink D, et al . Skin protection for photosensitized patients. Lasers Surg Med 2001;29:252-9.
[Google Scholar]
|
| 22. |
Lowe NJ, Friedlander F. In: Lowe, NJ, Shaath NA, Pathak MA, editors. Sunscreens: Development, evaluation and regulatory aspects, 2 nd ed. Marcel Dekker: New York; 1997. p. 35-58.
[Google Scholar]
|
| 23. |
Murphy G. Ultraviolet light and rosacea. Cutis 2004;74:13-6, 32-4.
[Google Scholar]
|
| 24. |
Skin protectant drug products for over-the counter human use. Fed Reg 1978;43:34641-2.
[Google Scholar]
|
| 25. |
Epstein JH. Biological effects of sunlight. In: Lowe, NJ, Shaath NA, Pathak MA, editors. Sunscreens: Development, evaluation and regulatory aspects, 2 nd ed. Marcel Dekker: New York; 1997. p. 83-100.
[Google Scholar]
|
| 26. |
Baldwin S, Odio MR, Haines SL, O'Connor RJ, Englehart JS, Lane AT. Skin benefits from continuous topical adminitration of a zinc oxide/petrolatum formulation by a novel disposable diaper. J Eur Acad Dermatol Venereol 2001;15:5-11.
[Google Scholar]
|
| 27. |
Cayrol C, Sarraute J, Tarroux R, Redoules D, Charveron M, Gall Y. A mineral sunscreen affords genomic protection against ultraviolet (UV) B and UVA radiation: In vitro and in situ assays. Br J Dermatol 1999;141:250-8.
[Google Scholar]
|
| 28. |
Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, et al . The potential risks of nano materials: A review carried out for ECETOC. Part Fibre Toxicol 2006;3:11.
[Google Scholar]
|
| 29. |
A review of the scientific literature on the safety of nanoparticulate titanium dioxide or zinc oxide in sunscreens. Box 100 Woden ACT 2606, OTC Medicines Section, Therapeutic Goods Administration, 2006. Available from: http://www.tga.gov.au/npmeds/sunscreen-zotd.pdf. [Last accessed on 2007 Jan 5].
[Google Scholar]
|
| 30. |
Muller R, Umbreit J, Liebetrau E, Nekwasil J. Reaction and effectiveness of prednisolone in zinc oxide containing dermatologic agents. Dermatol Monatsschr 1989;175:82-6.
[Google Scholar]
|
| 31. |
Timmins P, Gray EA. Degradation of hydrocortisone in a zinc oxide lotion. J Clin Hosp Pharm 1983;8:79-85.
[Google Scholar]
|
Fulltext Views
8,424
PDF downloads
4,246





