Translate this page into:
Moore Federman syndrome: A rare cause of pseudoscleroderma
Correspondence Address:
K Muhammed
Kunnummal House, Koroth School Road, Vadakara - 673 101, Kerala
India
| How to cite this article: Muhammed K, Nandakumar G. Moore Federman syndrome: A rare cause of pseudoscleroderma. Indian J Dermatol Venereol Leprol 2007;73:257-259 |
Abstract
The Moore Federman syndrome (MFS) is characterized by short stature, stiffness of the joints, characteristic facies and ocular abnormalities. Herein, we report the case of a 45 year-old lady with short stature, thickening of the skin, stiffness of the joints, typical facies, iridodonesis and cataract since the age of 12 years. She had short digits and no family history of similar illness. To the best of our knowledge, this could be the fourth report of MFS.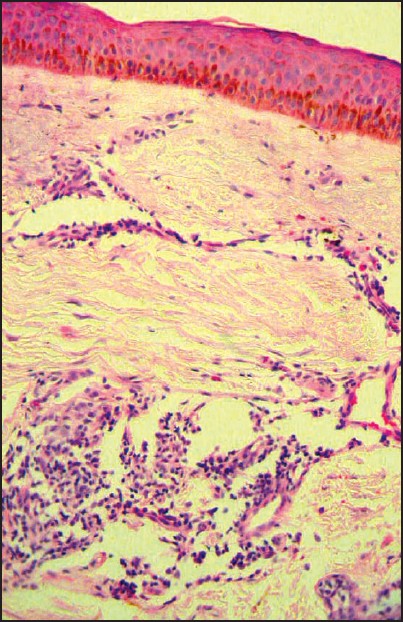 |
| Figure 4: Scleroderma-like changes on histopathological examination (H and E stain, x400) |
 |
| Figure 4: Scleroderma-like changes on histopathological examination (H and E stain, x400) |
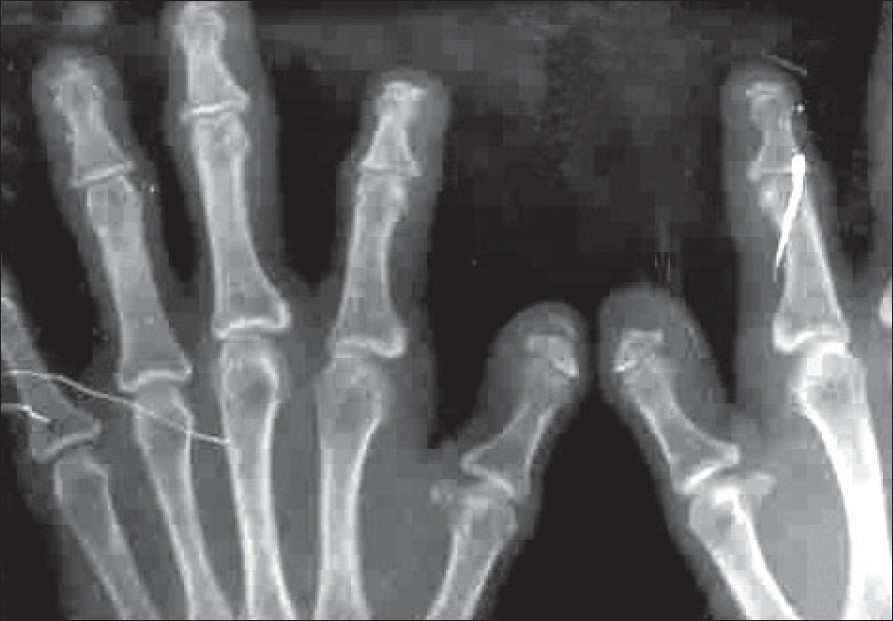 |
| Figure 3: X-ray of hands showing acro-osteolysis and loss of soft tissue of the terminal part of digits |
 |
| Figure 3: X-ray of hands showing acro-osteolysis and loss of soft tissue of the terminal part of digits |
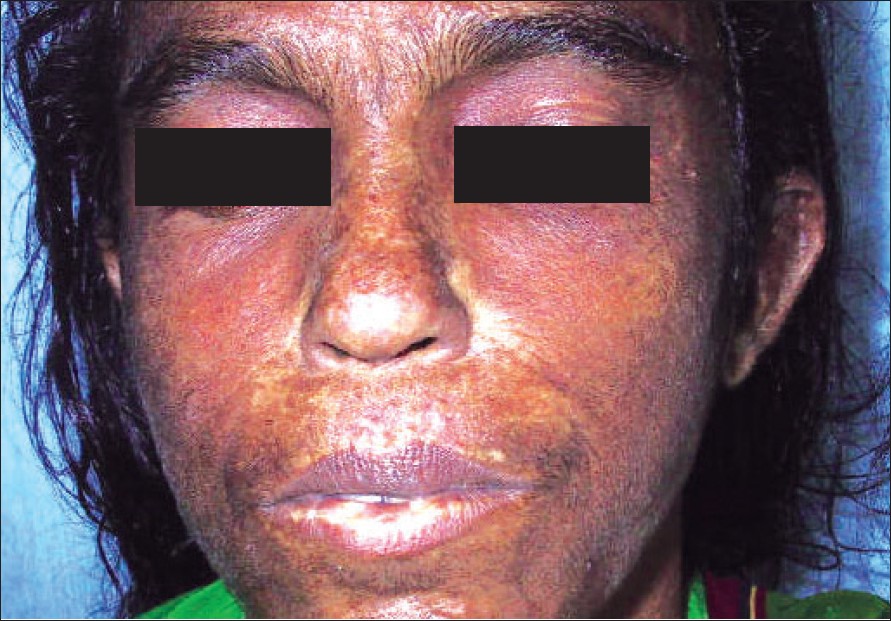 |
| Figure 2: Characteristic facies |
 |
| Figure 2: Characteristic facies |
 |
| Figure 1: Short and pudgy digits with which she cannot make a fist |
 |
| Figure 1: Short and pudgy digits with which she cannot make a fist |
Introduction
The Moore Federman syndrome (MFS), first reported in six members of the same family in 1965, is characterized by short stature, joint stiffness especially in the hands (those affected were unable to make a fist), normal intelligence, hypermetropia, glaucoma, asthma and hepatomegaly. [1] There were no subsequent reports of MFS for about 20 years.Hereditary conditions such as MFS, acromicric dysplasia (AMD) and geleophysic dysplasia (GPD) cause pseudoscleroderma. In 1989, Winter et al. pointed out the similarity between autosomal dominant MFS and AMD reported by Maroteaux in 1986. [2] The main features of AMD are short stature, short hands and feet and a characteristic facial appearance. The latter consists of round face, narrow palpebral fissures and a short stubby nose with anteverted nostrils. [2]
In 1993, Fell and Stanhope reported two more cases similar to MFS and AMD. [3] The term GPD was coined by Sprangler et al. because of the happy face of affected children (gelios= happy, physis=nature), for an entity characterized by short stature, multiple contractures, facial characteristics, skeletal symptoms of hands and feet and a focal accumulation of acid mucopolysacharides in the liver and heart. [4]
Most of the previous authors concluded that in spite of the different modes of inheritance, these three genetic disorders may be allelic forms of the same disease or different disturbances of the same metabolic pathway. [4] MFS is one of those rare conditions having the distinction of being dropped from the third edition of Smith′s ′Recognizable patterns of human malformation′. [5] We are reporting what is probably the fourth instance of this rare disease in a 45 year-old female with no family history. We also present a brief review of the literature reports of this condition.
Case Report
A 45 year-old, short-statured woman (height 146 cm) of normal intelligence, presented to us with features of tightening of the skin since the age of 12 years. She had short and pudgy digits since birth and was unable to make a fist [Figure - 1] with her hands. Her joints were stiff resulting in difficulty in walking and performing day-to-day activities. There was no history of dysphagia, exertional dyspnea, ′heart burn′ or Raynaud′s phenomenon. Her face, upper limbs and lower limbs showed sclerosis of the skin. She had a round face with narrow palpebral fissures and a small nose and she was unable to look upwards due to sclerosis of lids [Figure - 2].
All baseline hematological and urine examinations were unremarkable. The growth hormone levels and thyroid function tests were normal. The ANA test was negative. No organomegaly was detected clinically or sonologically in the abdomen. X-rays of the hands and feet showed evidence of acro-osteolysis with loss of soft tissue from the terminal parts of the digits as seen in scleroderma [Figure - 3]. Skin biopsy from the sclerosed area of the face showed scleroderma-like changes [Figure - 4]. There was no histological evidence of any storage diseases on special staining using alcian blue. Evaluation of the eyes showed iridodonesis, posterior and anterior subcapsular cataract and ectopia lentis. The patient was given reassurance regarding the disorder and symptomatic management was given.
Discussion
The MFS is a rare genetic cause for pseudoscleroderma. [6] Moore and Federman described it as familial dwarfism with stiff joints. [1] Even though MFS is an autosomal dominant condition, no other family members were affected in our case, which could be due to sporadic mutation. Our case had most of the features of MFS as described by Moore and Federman [1] and later by Winter et al. [2] Winter et al. reported four unrelated patients with short stature, stiffness of joints, short fingers, inability to make a fist and thickened skin on the forearm, with no evidence of lysosomal storage disorder. He raised the possibility that AMD may be the same as MFS. [2]
However, abnormal skin changes are less prominent in AMD and they may have laryngotracheal stenosis. [2] Faivre et al. described characteristic X-ray abnormalities of hands in their report of 22 patients with AMD and the joint limitation was occasional in these cases (13/22). [7] Several AMD patients with features of GPD and MFS, namely stiff joints, thickened skin and hepatomegaly have been reported, raising the question of whether GPD, MFS and AMD could be the same entity. [4],[7] The main differences seem to be the facial appearance, the aspect of the proximal femora and the storage phenomena. [7]
In all the three entities, a severe growth retardation is found although the severity is less in MFS. The joint contractures and facial dysmorphism are more marked in GPD and MFS. The femoral heads were reported to be large in GPD, deformed in MFS (not seen in our case) and deformed with a notched inside border in AMD. Geleophysic dysplasia is autosomal recessively inherited while MFS and AMD are autosomal dominant in inheritance. A few cases of AMD had X-linked dominant inheritance. [4],[7] The eye abnormalities and thickened skin were rare features [6] in AMD and GPD, which were conspicuous in our case.
In our patient, besides short metacarpals and phalanges, acro-osteolysis similar to scleroderma was noted. But the internal notch of the second metacarpal, external notch of the fifth metacarpal and delayed bone age which are characteristic of AMD were not seen in our case. The differences between AMD and MFS are minor and the existence of only two reports of MFS limit the knowledge of the disease. [7] In the previously reported cases, [1],[2],[3] histopathological findings of MFS and AMD were not mentioned. But in our case, the histopathological findings were consistent with scleroderma, suggesting pseudoscleroderma. [5]
The prognosis and life expectancy are usually unaltered, as in our case. The progressive thickening of the skin and the hepatomegaly in some cases suggests that the syndrome may indeed be due to an uncharacterized storage disease. [4] Fell and Stanhope treated their cases with growth hormone and had some response in children. [3] The original report of Moore and Federman was followed by the reports of similarity between AMD and MFS by Winter et al. and the description of Fell et al. To the best of our knowledge, this could be the fourth report of MFS.
| 1. |
Moore WT, Federman DD. Familial dwarfism and "stiff joints". Arch Intern Med 1965;115:398-404.
[Google Scholar]
|
| 2. |
Winter RM, Patton MA, Challencer J, Mueller RF, Baraister M. Moore-Federman Syndrome and acromicric dysplasia: Are they the same entity? J Med Genet 1989;26:320-5.
[Google Scholar]
|
| 3. |
Fell JM, Stanhope R. Reviving Moore-Federman syndrome. J Royal Soc Med 1993;86:52-3.
[Google Scholar]
|
| 4. |
Hennekam RC, van Bever Y, Oorthuys JW. Acromicric dysplasia and geleophysic dysplasia: Similarities and differences. Eur J Pediatr 1996;155:311-4.
[Google Scholar]
|
| 5. |
Smith DW. Recognizable patterns of human malformation. W.B Saunders: Philadelphia; 1982.
[Google Scholar]
|
| 6. |
Rowell NR, Goodfield MJ. The connective tissue diseases. In : Champion RH, Burton JL, Burn DA, Breathnach SM, editors. Textbook of Dermatology, 6 th edition. Blackwell Science: New York; 2000. p. 2512-3.
[Google Scholar]
|
| 7. |
Faivre L, Le Merrer M, Baumann C, Polak M, Chatelain P, Sulmont V, et al. Acromicric dysplasia: Long term outcome and evidence of autosomal dominant inheritance. J Med Genet 2001;38:745-9.
[Google Scholar]
|
Fulltext Views
3,894
PDF downloads
1,376





