Translate this page into:
Pembrolizumab-induced toxic epidermal necrolysis: A rare cause of severe adverse drug reaction
Corresponding author: Dr. Shekhar Neema, Armed Forces Medical College, Pune, Maharashtra, India. shekharadvait@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Sathu S, Vasudevan B, Shreshta S, Bhatt S, Lekshmipriya K. Pembrolizumab-induced toxic epidermal necrolysis: A rare cause of severe adverse drug reaction. Indian J Dermatol Venereol Leprol 2023;89:589–91.
Dear Editor,
Stevens-Johnson syndrome/toxic epidermal necrolysis is the most dreaded adverse cutaneous drug reaction and is potentially fatal, if not promptly managed.1 It is reported with medications such as non-steroidal anti-inflammatory drugs, anticonvulsants, antibiotics (beta-lactam), allopurinol and barbiturates; immune checkpoint inhibitors can also result in Stevens-Johnson syndrome/toxic epidermal necrolysis uncommonly. Pembrolizumab is a highly selective humanised monoclonal IgG4 antibody, directed against the programmed cell death-1 protein receptor on the cell surface. It is being increasingly used to treat unresectable/metastatic malignancies, melanoma and lymphoma and has shown good efficacy.2 We present a case of pembrolizumab-induced toxic epidermal necrolysis in a patient with metastatic squamous cell carcinoma of the penis.
A 55-year-old male, a known case of moderately differentiated squamous cell carcinoma of the penis, post-chemo-radiotherapy, was diagnosed with progressive disease and metastases to the heart, lung and liver. He was being treated with oral gefitinib 250 mg, an epidermal growth factor receptor (EGFR) inhibitor, once daily for the last three months. However, in view of progressive metastatic disease, gefitinib was stopped; pembrolizumab 200 mg was administered as an intravenous infusion and was planned as three weekly injections. Two weeks later, he developed fever, redness of eyes, reddish rash on the trunk and painful oral ulcers. General and systemic examinations were within normal limits. Dermatological examination showed widespread erythematous to purpuric macules and a few bullae and erosions involving the face, trunk and extremities, constituting approximately 45% of body surface area [Figure 1]. Oral mucosa showed multiple erosions and there was haemorrhagic crusting of the lips. Bilateral conjunctiva showed chemosis [Figure 2]. A provisional diagnosis of toxic epidermal necrolysis was made with toxic epidermal necrolysis specific severity of illness score (SCORTEN) of the patient being 4 out of 7 (one point each for age >40 years; malignancy, epidermal detachment >10% and blood urea 42 mg/dL), indicating significant (60%) risk of mortality. The score on Naranjo adverse drug reaction probability scale was 6 indicating a “probable” association with pembrolizumab. Baseline haematological and biochemical parameters were within normal limits except for blood urea nitrogen (42 mg/dL) and serum creatinine (1.8 mg/dL). Histopathology of skin showed acantholysis, basal keratinocyte apoptosis and sub-epidermal bulla formation with inflammatory infiltrate of neutrophils and lymphocytes in the dermis. Direct immunofluorescence was negative for immunoglobulin G (IgG), immunoglobulin M (IgM), immunoglobulin A (IgA) and complement 3 (C3). No viral cytopathic changes were seen. Pembrolizumab was withheld and cyclosporine 250 mg (5 mg/kg) in two divided doses was started. The patient continued to develop new lesions after 72 hours and was given intravenous immunoglobulin in a dose of 2 gm/kg body weight over two days.3 Cyclosporine was continued for 10 days in the same dose. The patient responded well with no new lesions and healing of pre-existing erosions. However, the patient succumbed to complications of toxic epidermal necrolysis in the form of sepsis with multi-organ dysfunction syndrome and underlying metastatic carcinoma after about two weeks despite all possible care.
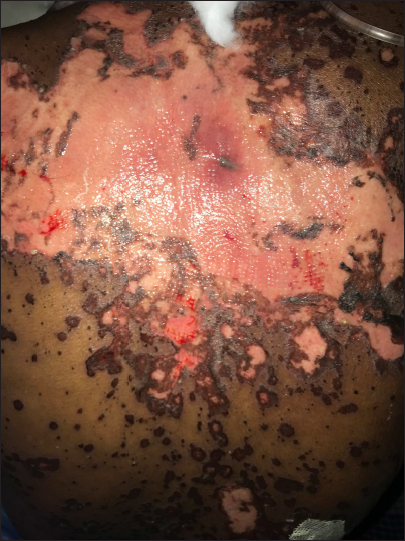
- Pembrolizumab-induced toxic epidermal necrolysis showing involvement of trunk with sheet-like detachment of the skin
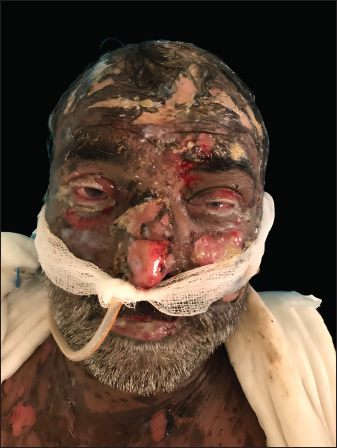
- Pembrolizumab-induced toxic epidermal necrolysis showing erosions involving face, conjunctival congestion, chemosis and crusting over the lips
Pembrolizumab is a monoclonal antibody against programmed cell death-1 protein receptors on the cell surface. Programmed cell death-1 protein inhibitors produce durable responses in patients with advanced and metastatic squamous cell carcinoma.4 It belongs to the group of immune checkpoint inhibitors and acts by enhancing the intrinsic ability of the immune system to destroy tumour cells and by doing so they also alter the immune tolerance and homeostasis leading to untoward effects, termed immune-related adverse events. Skin is a common site for these immune-related adverse events and can manifest as pruritus, morbilliform, psoriasiform, lichenoid, eczematous, immunobullous reactions and vitiligo.5 Rarely severe cutaneous adverse drug reaction like Stevens-Johnson syndrome/toxic epidermal necrolysis has also been reported. In addition to the skin, the mucosae (conjunctival, oral and genital) may also be significantly affected with the possibility of complications like blindness.6 Exact mechanisms for the occurrence of life-threatening reactions like Stevens-Johnson syndrome/toxic epidermal necrolysis is unclear. Goldinger et al. suggested that the immune checkpoint inhibitors cause activation and proliferation of auto-reactive CD8+ T cells targeting keratinocytes with self-antigens leading to the occurrence of cutaneous drug reactions.2
Stevens-Johnson syndrome/toxic epidermal necrolysis following drugs like non-steroidal anti-inflammatory drugs (NSAIDS), anticonvulsants, antibiotics, allopurinol etc, occur acutely, usually within two weeks of administration of the culprit drug. However, the same reaction with pembrolizumab was reported to have a delayed onset (median of three weeks) and a prolonged course.7 This atypical presentation could be explained by the pharmacokinetics of the drug. For example, anticonvulsant lamotrigine has a mean half-life of approximately 23–37 hours while pembrolizumab has a half-life of 23 days and takes a longer time to reach steady states, hence a delayed onset of reaction. In addition to the longer half-lives, the underlying mechanism of Stevens-Johnson syndrome/toxic epidermal necrolysis with commonly implicated drugs involve activation and proliferation of drug-specific T cells, occurring rapidly following drug exposure, while the process with checkpoint inhibitors like pembrolizumab is complex and involves proliferation of T cells directed against self-antigens in the skin, loss of peripheral immune tolerance before these reactions can fully manifest.8 The cases of pembrolizumab-induced Stevens-Johnson syndrome/toxic epidermal necrolysis and toxic epidermal necrolysis published in the literature have been tabulated in Table 1.
| Reference | Diagnosis | Age | Sex | Malignancy | Concomitant medications | Onset | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Cai et al.9 | TEN | 63 | Male | Metastatic adenocarcinoma lung | Nil | 3 days after first cycle | Corticosteroid Cyclosporine |
Recovered |
| Robinson et al.7 | SJS/TEN | 55 | Female | Metastatic cervical squamous cell carcinoma | Nil | 17 days after first cycle | Methylprednisolone | Recovered |
| Chow et al.10 | TEN | 63 | Male | Metastatic lung adenocarcinoma | Perindopril, oxycodone, levetiracetam | 17 days after 3rd cycle | Methylprednisolone, IVIG, cyclosporine | Recovered |
| Kian et al.11 | TEN | 65 | Male | Metastatic non-small cell lung carcinoma | Perindopril, amlodipine | 3 days after first cycle | Recovered | |
| Marin et al.12 | TEN | 77 | Male | Metastatic esophageal adenocarcinoma | Folinic acid Fluorouracil Oxaliplatin Trastuzumab |
10 days after first cycle | Methylprednisolone cyclosporine | Recovered |
| Storandt et al.13 | SJS/TEN | 55 | Female | Adenocarcinoma lung | Pemetrexed | 6 months after first cycle | Methylprednisolone IVIG |
Recovered |
| Aoyama et al.14 | TEN | 72 | Female | Carcinoma lung | Pemetrexed Carboplatin celecoxib | 14 days after second cycle | Methylprednisolone IVIG |
Recovered |
| Choi et al.15 | TEN | 62 | Male | Urothelial carcinoma | Nil | 7 days after first cycle | Methylprednisolone | Recovered |
| Cao et al.16 | SJS/TEN | 69 | Male | Carcinoma esophagus | Oxaliplatin Gimeracil Tegafur Oteracil |
2 weeks after first cycle | Methylprednisolone IVIG Plasmapheresis |
Recovered |
| Oguri et al.17 | SJS/TEN | 76 | Male | Carcinoma lung | Denosumab Radiation |
2 weeks after first cycle | Methylprednisolone IVIG |
Fatal |
| Kumar et al.18 | TEN | 57 | Female | Carcinoma lung | Nil | 2 weeks after first cycle | Methylprednisolone Plasmapheresis Infliximab |
Fatal |
SJS: Stevens-Johnson syndrome, TEN: Toxic epidermal necrolysis, IVIG: Intravenous immunoglobulin
Correct identification of culprit drug and its withdrawal, supportive care and administration of cyclosporine, steroids or intravenous immunoglobulin are the mainstay of treatment for reactions caused by usual drugs. However, the longer half-lives of immune checkpoint inhibitors and a different underlying mechanism usually cause a delayed and unpredictable response to treatment with a significantly increased risk of mortality (approximately 60%) as reported in various case reports. Plasmapheresis may be a useful treatment option, especially in cases with progressive rash and poor response to conventional treatment, considering the longer half-lives of these molecules.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Stevens-Johnson syndrome and toxic epidermal necrolysis: 11-year demographic clinical and prognostic characteristics. Indian J Dermatol. 2022;67:12-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. 2016;22:4023-9.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: Multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol. 2003;139:26-32.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: A retrospective analysis from the University of Southern California. J Cancer Res Clin Oncol. 2021;147:1803-11.
- [CrossRef] [PubMed] [Google Scholar]
- Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158-68.
- [CrossRef] [PubMed] [Google Scholar]
- Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int J Dermatol. 2020;59:E183-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pembrolizumab-induced Stevens–Johnson syndrome/toxic epidermal necrolysis in a patient with metastatic cervical squamous cell carcinoma: A case report. Am J Dermatopathol. 2020;42:292-6.
- [CrossRef] [PubMed] [Google Scholar]
- Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res. 2018;10:1259-73.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis associated with pembrolizumab. J Oncol Pharm Pract. 2020;26:1259-65.
- [CrossRef] [PubMed] [Google Scholar]
- Pembrolizumab-induced toxic epidermal necrolysis: Case report. Oxf Med Case Reports. 2022;2022:omac025.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous immunoglobulin efficacy on pembrolizumab induced severe toxic epidermal necrolysis. Anticancer Drugs. 2022;33:e738-40.
- [CrossRef] [PubMed] [Google Scholar]
- Pembrolizumab-induced toxic epidermal necrolysis in a patient with metastatic esophageal adenocarcinoma. R I Med J (2013). 2022;105:34-6.
- [PubMed] [PubMed Central] [Google Scholar]
- A case of Stevens-Johnson syndrome/toxic epidermal necrolysis in a patient receiving chemo-immunotherapy with pemetrexed and pembrolizumab. CPC: Case Reports. 2021;3:100048.
- [CrossRef] [Google Scholar]
- Toxic epidermal necrolysis as a complication of pembrolizumab treatment in a lung cancer patient. J Cutan Immunol Allerg. 2021;4:41-2.
- [CrossRef] [Google Scholar]
- A case of pembrolizumab-induced toxic epidermal necrolysis. Korean J Dermatol. 2022;60:120-4.
- [Google Scholar]
- Pembrolizumab-induced autoimmune Stevens-Johnson syndrome/toxic epidermal necrolysis with myositis and myocarditis in a patient with esophagogastric junction carcinoma: A case report. Transl Cancer Res. 2021;10:3870-6.
- [CrossRef] [PubMed] [Google Scholar]
- A case of Guillain-Barré syndrome and Stevens-Johnson syndrome/toxic epidermal necrosis overlap after pembrolizumab treatment. J Investig Med High Impact Case Rep. 2021;9:23247096211037462.
- [CrossRef] [PubMed] [Google Scholar]
- Pembrolizumab induced toxic epidermal necrolysis. Curr Probl Cancer. 2020;44:100478.
- [CrossRef] [PubMed] [Google Scholar]





